Adding Bevacizumab to Carboplatin/ Paclitaxel Regimen Improves Survival in Patients With Advanced NSCLC
This supplement to Oncology News International includes more than 15 reportson presentations made at the 41st annual meeting of the American Society of Clinical Oncology.Reviews focus on the use of targeted agents in non–small-cell lung cancer and other solid tumors,evaluating the novel therapies bevacizumab, cetuximab, bortezomib, erlotinib, and gefitinib, aloneand/or in combination with other chemotherapy agents. Continuing medical education credit isavailable by completing a post-test and evaluation online at www.cancernetwork.com/cme.
ORLANDO, Florida-Bevacizumab(Avastin) combined with chemotherapyextends survival in advanced,nonsquamous, non-small-celllung cancer (NSCLC), results of a randomized,multisite trial that tested thecombination as first-line therapy show.The findings are expected to changethe standard of care, said the investigatorswho presented their findingsat the 41st annual meeting of theAmerican Society of Clinical Oncology(ASCO) (abstract LBA4).New Standard of CareBevacizumab with carboplatin andpaclitaxel "is now the ECOG referencestandard for the first-line treatment ofadvanced nonsquamous NSCLC," saidAlan Sandler, MD, associate professorof medicine at Vanderbilt-IngramCancer Center, who led the trial forthe Eastern Cooperative OncologyGroup (ECOG).The trial enrolled 878 patients withstage IIIb or IV disease and randomizedthem to one of two arms. Onearm received paclitaxel at 200 mg/m2and carboplatin to an AUC of 6 on day1 every 3 weeks for six cycles, while theother arm received these two drugsplus bevacizumab at 15 mg/kg on day1 for six cycles, followed by bevacizumabalone until disease progressionor intolerable toxicity. Patients withsquamous cell carcinoma were notincluded in the trial because the risk ofserious hemorrhage with bevacizumabis greater in this group.Better OutcomesPatients in the bevacizumab armhad significantly better outcomes.Median survival in this group was 12.5months, vs 10.2 months for the groupthat received chemotherapy alone. Theresponse rate in the bevacizumabgroup was 27.2%, vs 10% for the controlgroup. Median time to progressionwas 6.4 months vs 4.5 months,respectively.
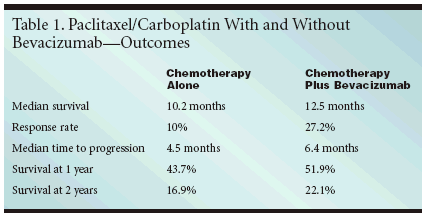
At 1 year, 51.9% of patients in thebevacizumab group were alive, comparedwith 43.7% in the control group.At 2 years, 22.1% of the bevacizumabpatients were alive vs 16.9% of thosewho received chemotherapy alone.Well-Tolerated TxBoth regimens were well tolerated,Dr. Sandler said, although toxicity wasgreater on the bevacizumab arm.Grade 4/5 neutropenia was experiencedby 24% of the bevacizumab patientsvs 16.4% of those on the controlarm. Hemorrhage occurred in 4.5% ofthe patients receiving bevacizumaband in 0.7% of the patients receivingchemotherapy alone. There were 10treatment-related deaths, 5 of whichwere caused by hemoptysis and occurredon the bevacizumab arm. Hypertensionwas significantly greateramong those taking bevacizumab-6% vs 0.7%-but was easily managedwith medications, Dr. Sandler said.Bevacizumab is a humanized monoclonalantibody that targets vascularendothelial growth factor (VEGF) andis thought to inhibit the growth ofblood vessels feeding the tumor. LeeEllis, MD, professor of surgical oncologyand cancer biology at The Universityof Texas M.D. Anderson CancerCenter, Houston, discussed the abstractand pointed out that its mechanismof action is still uncertain. "Anti-VEGF therapy inhibits activity of bothendothelial cells and some tumorcells," he said in his discussion of theresults."We do think that anti-VEGFCenter, Houston, discussed the abstractand pointed out that its mechanismof action is still uncertain. "Anti-VEGF therapy inhibits activity of bothendothelial cells and some tumorcells," he said in his discussion of theresults. "We do think that anti-VEGFtherapy is antiangiogenic, though thisis very difficult to prove in clinicaltrials, and I think we will have a hardtime proving this in the future. Butefficacy is what counts."Dr. Ellis added that it would bevery useful to develop predictive markersfor bevacizumab, not only for outcomebut also for adverse events. "Weneed to be cognizant of the adverseeffects," he emphasized, "includinghypertension, bowel perforation, andhemorrhage. Although infrequent,they are important adverse events."