Cetuximab Shows Efficacy in Advanced Non–Small-Cell Lung Cancer
This supplement to Oncology News International includes more than 15 reportson presentations made at the 41st annual meeting of the American Society of Clinical Oncology.Reviews focus on the use of targeted agents in non–small-cell lung cancer and other solid tumors,evaluating the novel therapies bevacizumab, cetuximab, bortezomib, erlotinib, and gefitinib, aloneand/or in combination with other chemotherapy agents. Continuing medical education credit isavailable by completing a post-test and evaluation online at www.cancernetwork.com/cme.
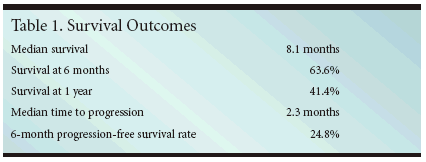
ORLANDO, Florida-Cetuximab (Erbitux) showed activityin patients with advanced non-smallcelllung cancer (NSCLC) who hadreceived prior chemotherapy, includingplatinum therapy, in a recentlycompleted phase II trial. The efficacyof cetuximab was comparable to thatof other agents in this population, accordingto lead investigator RogerioC. Lilenbaum, MD, director of thethoracic oncology program at MountSinai Comprehensive Cancer Centerin Miami Beach, Florida (abstract7036).For the study, patients received aninitial dose of cetuximab at 400 mg/m2on day 1 of a 4-week cycle, followed byweekly doses of 250 mg/m2 until diseaseprogression or unacceptable toxicityoccurred.Good Efficacy, TolerabilityDr. Lilenbaum and colleagues reportedthree partial responses amongthe 60 evaluable patients. Median timeto progression was 2.3 months andmedian survival time was 8.1 months.After 6 months, 63.6% of the participantswere still alive, and 41.4% werealive after 1 year.
"I think this does demonstrate efficacy,"said discussant Charles Rudin,MD, PhD, director of the lung cancertherapeutics program at Sidney KimmelComprehensive Cancer Center."The survival rates are reasonablygood," he said, and "consistent withour current standard of care."The drug was well tolerated. Theprincipal adverse effects were the flulikesymptoms commonly seen withcetuximab, and rash, the investigatorssaid. None of the patients discontinuedthe drug because of toxicities.Distinct Mechanism of ActionCetuximab is a monoclonal antibodythat has been approved by theFood and Drug Administration fortreatment of colorectal cancer. Likegefitinib (Iressa) and erlotinib (Tarce-va), which are tyrosine kinase (TK)inhibitors, it targets the epidermalgrowth factor receptor (EGFR), butits mechanism of action is thought tobe different. Cetuximab appears towork by binding to EGFR, therebyblocking growth factors from bindingto the receptor and initiating cellgrowth.Because of the discovery last yearthat the activity of TK inhibitors islinked to mutations in EGFR, Dr.Lilenbaum and his colleagues lookedfor an association between these mutationsand the response to cetuximab.No clear relationship emerged. Tissuesamples from 3 of 39 patients hadmutations, all in patients with stabledisease; tissue samples were availablefor two of the three responders, andthese were negative for mutations.This finding suggests "that the activityspectrum of cetuximab is likelyto be distinct from that of EGFR TKinhibitors," Dr. Rudin said. "We needto begin to look at molecular determinantsof cetuximab activity," he added.Next steps for cetuximab may includetesting in combination with chemotherapyor other targeted drugs.Dr. Rudin suggested cetuximab witherlotinib and bevacizumb (Avastin),in particular. "I think that this wouldbe a very interesting combination," hesaid.