Smoking History May Prove Useful in Treatment Planning for NSCLC Patients
This supplement to Oncology News International includes more than 15 reportson presentations made at the 41st annual meeting of the American Society of Clinical Oncology.Reviews focus on the use of targeted agents in non–small-cell lung cancer and other solid tumors,evaluating the novel therapies bevacizumab, cetuximab, bortezomib, erlotinib, and gefitinib, aloneand/or in combination with other chemotherapy agents. Continuing medical education credit isavailable by completing a post-test and evaluation online at www.cancernetwork.com/cme.
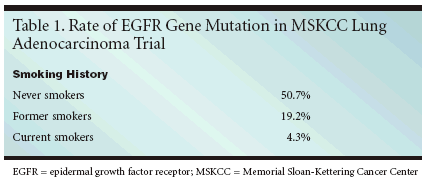
ORLANDO-A lung cancerpatient's smoking history may helppredict his or her response to gefitinib(Iressa) and erlotinib (Tarceva), accordingto two studies. If follow-upstudies turn out as hoped, smokinghistory could become a useful tool forselecting treatments for patients withnon-small-cell lung cancer (NSCLC),investigators reported.In one study (abstract 7069), theinvestigators sequenced the epidermalgrowth factor receptor (EGFR) genein 265 tumor samples from patientswith lung adenocarcinomas, lookingfor mutations that have been associatedwith response to gefitinib and erlotinib.They then correlated the presenceof the mutations with eachpatient's smoking history.The rate of mutations in neversmokers was 50.7%, in former smokers,19.2%, and in current smokers,4.3%, reported lead investigator DuyKhanh Pham, MD, a researcher in theDepartment of thoracic surgery, MemorialSloan-Kettering CancerCenter in New York. EGFR mutationswere present in tumors from patientswith a smoking history of 15 or fewerpack years; in fact the incidence ofmutations in these former smokersdid not differ from the incidence ofmutations in never smokers, Dr. Phamtold ONI. Patients who had beensmoke-free for more than 25 yearsalso were likely to have mutations.
The investigators concluded that athorough smoking history can assistclinicians in assessing the probabilityof the presence of EGFR mutationsand, consequently, in estimating thelikelihood of response to treatmentwith gefitinib or erlotinib.15 Pack-Year CutoffIn discussing the trial, RomanPerez-Soler, MD, chief of the oncologydivision at Montefiore MedicalCenter, New York, agreed that the findingshad potential clinical implications.Particularly interesting, he said,were the data indicating that 15 packyears could be a cutoff point for thepresence of EGFR mutations. "I thinkthat's an important and potentiallyuseful finding..." he said. "It might bethat [the 15 pack-year cutoff] wouldbe a better way to select patients."Dr. Perez-Soler suggested that datafrom the National Cancer Institute ofCanada's landmark BR.21 trial witherlotinib could be used to confirmthese findings. "Can we validate this? Ithink so," he said, recommending thatthe BR.21 results be analyzed usingthat particular cutoff.Smoking and BR.21 DataAlthough the BR.21 data may nothave been analyzed yet according tothe smokers' number of pack years,one group of investigators has examinedthe smoking history of BR.21patients in relation to EGFR expression(abstract 7033). Gary Clark, PhD,and colleagues at OSI Pharmaceuticalsin Boulder, Colorado, found thatsmoking history was more predictiveof a survival benefit from erlotinibthan whether a patient overexpressedEGFR.These investigators looked at 311BR.21 patients whose EGFR status andsmoking history was known. Thosewho had never smoked were morelikely to respond to erlotinib than thosewho were former or current smokers,although there were responses in allthree categories. By contrast, there wasno significant correlation betweenEGFR expression and response, saidDr. Clark, who is vice president forbiostatistics and data management atOSI.Dose-EscalationStudy PlannedOne reason for the lower responserate in current smokers may be thatnot enough erlotinib reaches their tumors,Dr. Clark said. He added that"according to this hypothesis, erlotinibis cleared from the body morequickly in smokers, as smoking isknown to upregulate enzymes CYP1A1and CYP1A2, which speed the clearanceof erlotinib. If true, higher dosesof erlotinib might compensate for theincreased clearance, and physicianscould prescribe higher doses of thedrugs for smokers in order to achievethe desired exposure." OSI is planninga dose-escalation study that will testthis idea, he said.Commenting on this study, ManuelHidalgo, MD, of the Johns HopkinsSchool of Medicine, Baltimore, said itwas notable for the large number ofpatients. This makes it a welcome confirmationof smaller studies that haveshown a higher response rate to tyrosinekinase inhibitors in nonsmokers,he added.