Adding Cetuximab to Cisplatin/Vinorelbine Raises Response Rate in Phase II NSCLC Trial
This special supplement toOncology News International presents11 reports on novel agents targetingHER1/EGFR, VEGF, and HER2/neu receptorsin the treatment of non–small-cell lung cancer,colorectal cancer, mesothelioma, andglioblastoma. The reports summarizeselected presentations from theAmerican Society of Clinical Oncology (ASCO)39th Annual Meeting and a satellitesymposium held in conjunction with ASCO.
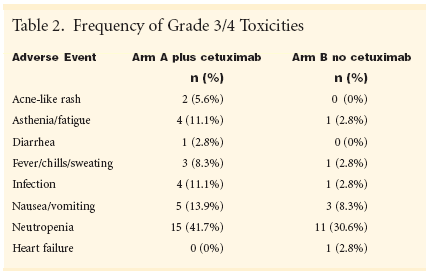
HAMBURG, Germany-Adding cetuximab (Erbitux) to a standardchemotherapy regimen of cisplatinand vinorelbine (Navelbine) wassafe and produced "promising activity"against non-small-cell lung cancer(NSCLC) in interim phase II resultsfrom the Lung Cancer CetuximabStudy (LUCAS).Investigator Ulrich Gatzemeier,MD, of Grosshansdorf Hospital inHamburg reported an overall responserate of 53.3% among 30 patients giventhe standard regimen plus cetuximabvs 32.3% for 31 patients given only thestandard regimen (ASCO abstract2582). The cetuximab arm included 1complete responder and 15 partialresponders. No complete responsesand 10 partial responses were recordedin the standard therapy arm(see Table 1).Conversely, progressive disease wasmore than three times higher for patientsreceiving standard therapy withoutcetuximab. Seven patients (22.6%)in the standard therapy arm had progressivedisease vs two patients (6.7%)in the cetuximab arm. Stable diseasewas also slightly higher in the standardtherapy arm, 14 patients (45.2%),compared to 12 (40%) in the cetuximabarm."We hope that we can increase theresponse rate with the addition ofcetuximab to a two-drug combination,"Dr. Gatzemeier told ONI.Until now, phase III trials adding athird drug to the standard cisplatin/vinorelbine combination have failedto boost response rates for NSCLCbeyond 26% to 30% or extend themedian time to progression beyond 4months. The LUCAS study did notreport data on duration of response ortime to progression.Dr. Gatzemeier cautioned that datareported are only interim. The trialopened enrollment early in 2002, andaccrual of 40 patients in each arm iscomplete, he said, forecasting finalresults would be ready by the end ofthe year.Researchers at hospitals in Spain,Poland, and France and at Merck KgaAin Darmstadt, Germany, are collaboratingin the study. Merck licensescetuximab from ImClone Systems,Inc., a US-based biotechnology company.Synergy in Lab Models
A monoclonal antibody, cetuximabtargets the HER1/epidermal growthfactor receptor (HER1/EGFR), whichis highly expressed in most advancedcases of NSCLC. It has shown synergisticactivity with cisplatin and vi-norelbine in laboratory tumor models,according to the investigators.Inclusion criteria included diagnosisof histologically confirmed stageIIIB or IV NSCLC with documentedmalignant pleural effusion, immunohistologicalevidence of EGFR-expressionin the primary tumor, and at leastone metastasis and Karnofsky performancestatus ≥ 70. Patients with previouschemotherapy, monoclonal antibodyexposure, or EGFR targetingtherapy were excluded.All patients received a standardregimen of 80 mg/m2 of cisplatin and25 mg/m2 of vinorelbine on days 1 and8 in 3-week cycles. Half the patientsalso received an initial infusion of400 mg/m2 of cetuximab followed by250 mg/m2 weekly. Response is assessedevery two cycles by CT scanuntil progression.
No Apparent Toxicity Increase
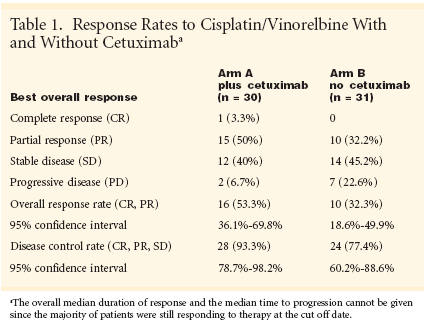
Although 61% of patients in theexperimental arm had grades 3/4 adverseevents and seven were withdrawnfrom the study, the investigators reportedthat cetuximab did not appearto increase chemotherapy toxicity.Among patients who received the standardregimen without cetuximab,69.4% had grades 3/4 adverse eventsand four could not continue on study.The most common adverse eventsin both groups were neutropenia followedby nausea/vomiting. Two patientson cetuximab experienced rash,which other researchers have correlatedwith response to EGFR-targetingagents (see Table 2)."The safety profile at this point isgood, without major increase of toxicityregarding cytotoxic chemotherapy,"Dr. Gatzemeier said. "Adverseevents like rash and the relation toresponse have to be analyzed."