Trastuzumab Shows Limited Promise in Non-Small-Cell Lung Cancer
This special supplement toOncology News International presents11 reports on novel agents targetingHER1/EGFR, VEGF, and HER2/neu receptorsin the treatment of non–small-cell lung cancer,colorectal cancer, mesothelioma, andglioblastoma. The reports summarizeselected presentations from theAmerican Society of Clinical Oncology (ASCO)39th Annual Meeting and a satellitesymposium held in conjunction with ASCO.
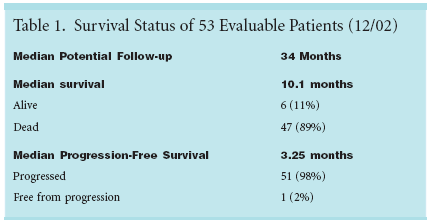
PHILADELPHIA-Matureresults from a multicenter phase IIstudy by the Eastern Cooperative OncologyGroup (ECOG) suggest trastuzumab(Herceptin) is safe and moderatelypromising in combination withpaclitaxel and carboplatin (Paraplatin)for some patients with non-small-celllung cancer (NSCLC).Whether the investigation willprogress to a phase III trial is unclear,however, as the researchers are uncertainas to how many lung cancer patientsare positive for the HER2/neumutation targeted by trastuzumab."The problem is, do we have enoughpatients to mount a study? That is thebig issue," investigator Corey Langer,MD, FACP, director of thoracic oncologyat Fox Chase Cancer Center,told ONI.The trastuzumab-chemotherapycombination produced a median survivalof 10.1 months and a medianprogression-free survival of 3.25months (see Table 1), according to areport by Dr. Langer (ASCO abstract2606). At a median follow-up of 34months, 6 of 53 evaluable patients werestill alive. Only one was free from progression.
Across-the-Board Response
Response rates showed that 13 ofthese patients (24.5%) had partial responses,21 (39.6%) had stable disease,and 16 (28.3%) had progressivedisease (see Table 2). The results are"as good as if not better than what wetypically see," Dr. Langer said, adding,"The interesting thing is response occurredacross the board. I can't say if adegree of HER2 positivity translatedto a better response rate."From August 1999 to May 2000,the investigators screened 139 patients,of whom 82 proved to be HER2 positive.All had advanced NSCLC. Eligi-bility criteria included recurrent, stageIV and stage IIIB (wet) disease and anECOG performance status of 0 to 1.Prior chemotherapy was not allowed.
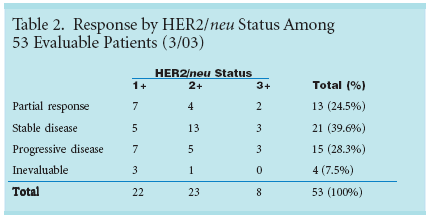
Of the 56 patients initially enrolled,53 turned out to be eligible. Amongthose evaluated, only eight were at theHER2 level of 3+, which Dr. Langersaid is used to justify use of trastuzumabin breast cancer. The remainderwere closely split with 22 patientsat 1+ and 23 patients at 2+.The proportion of HER2-positivelung cancer patients is probably lowerthan the proportion in the populationscreened for this study, according toDr. Langer, as oncologists did not referpatients known to be negative forthe mutation. Only five patients hadsquamous histology, another indicationthat the HER2 mutation might beuncommon among lung cancer patients.Subgroup Could Benefit
Nonetheless, a subgroup of patientsmight benefit, according to Dr. Langer,depending on what the median survivalis for patients with HER2/neu.That figure is currently unknown. IfHER2/neu patients have a median survivalof 5 months with standard chemotherapy,10 months would be asignificant increase, Dr. Langer noted.If their median survival is on the orderof 8 months, however, the addedmonths suggest less than the potentialof some other novel agents also inearly trials.Patients in the phase II study receivedan intravenous loading dose of4 mg/kg of trastuzumab followed by2 mg/kg weekly. Paclitaxel and carboplatinwere delivered in 3-week cycles.Toxicity appeared similar to chemotherapyalone, and Dr. Langer said thefew cases of left ventricular ejectionfraction decreases were so trivial thatthis need not be monitored if patientsare not receiving anthracycline in futuretrials.Ultimately, he predicted that noone novel agent but a combination oftherapies will prove most effective."Lung is a multihit malignancy. Manydifferent genetic abnormalities conspireto create a cancer," Dr. Langersaid, adding, "The bottom line is wereally don't know what the real targetsare, or how drugs interact. We're reallyin our infancy in understanding thiswhole field."