Adjuvant Chemotherapy for Early-Stage Breast Cancer: The tAnGo Trial
The tAnGo trial is a randomized, open-label, multicenter phase IIItrial examining adjuvant treatment with epirubicin (Ellence)/cyclophosphamide(Cytoxan, Neosar) for four cycles followed by paclitaxel aloneor combined with gemcitabine (Gemzar) for four cycles in patients withearly-stage breast cancer. In the Cancer and Leukemia Group B(CALGB) 9344 trial, addition of paclitaxel to anthracycline/cyclophosphamideadjuvant therapy resulted in increased time to recurrence andimproved survival. Because an unplanned subgroup analysis in CALGB9344 indicated a significant benefit of paclitaxel in patients with estrogenreceptor (ER)-negative disease but not ER-positive disease, the initialtAnGo trial design called for enrollment of patients with ER-negativedisease. The tAnGo trial entry criteria were recently amended toallow any ER status, given experience suggesting that clinical benefitof taxane-containing regimens in ER-positive disease may emerge overa time frame longer than that required to detect benefit in ER-negativedisease. Gemcitabine has been included as a partner for paclitaxel inthe tAnGo trial based on high response rates, including high completeresponse rates, observed in phase II trials of the combination in moreadvanced disease and based on the tolerability and safety of the combinationcompared with those of other taxane-containing two-drug combinations.The tAnGo trial is currently accruing patients and has atarget population of 3,000. Trial results should provide important informationon the role of gemcitabine in adjuvant therapy for breastcancer.
The tAnGo trial is a randomized, open-label, multicenter phase III trial examining adjuvant treatment with epirubicin (Ellence)/cyclophosphamide (Cytoxan, Neosar) for four cycles followed by paclitaxel alone or combined with gemcitabine (Gemzar) for four cycles in patients with early-stage breast cancer. In the Cancer and Leukemia Group B (CALGB) 9344 trial, addition of paclitaxel to anthracycline/cyclophosphamide adjuvant therapy resulted in increased time to recurrence and improved survival. Because an unplanned subgroup analysis in CALGB 9344 indicated a significant benefit of paclitaxel in patients with estrogen receptor (ER)-negative disease but not ER-positive disease, the initial tAnGo trial design called for enrollment of patients with ER-negative disease. The tAnGo trial entry criteria were recently amended to allow any ER status, given experience suggesting that clinical benefit of taxane-containing regimens in ER-positive disease may emerge over a time frame longer than that required to detect benefit in ER-negative disease. Gemcitabine has been included as a partner for paclitaxel in the tAnGo trial based on high response rates, including high complete response rates, observed in phase II trials of the combination in more advanced disease and based on the tolerability and safety of the combination compared with those of other taxane-containing two-drug combinations. The tAnGo trial is currently accruing patients and has a target population of 3,000. Trial results should provide important information on the role of gemcitabine in adjuvant therapy for breast cancer.
The tAnGo trial (paclitaxel, Anthracycline, Gemcitabine [Gemzar], and cyclophosphamide [Cytoxan, Neosar]) is a randomized, open-label, phase III trial designed to determine the role of gemcitabine in paclitaxel-containing epirubicin (Ellence)-based adjuvant chemotherapy for early-stage breast cancer. The combination of paclitaxel/ gemcitabine following epirubicin/ cyclophosphamide is a particularly attractive treatment option, given the responses observed in anthracyclinepretreated patients with more advanced disease who received the combination. Paclitaxel Plus Anthracycline/ Cyclophosphamide: Effect of CALGB on the tAnGo Trial Design The inspiration and rationale for the initial tAnGo trial design were provided partly by the results of the Cancer and Leukemia Group B (CALGB) 9344 trial, which added paclitaxel to standard doxorubicin/cyclophosphamide adjuvant therapy.[1] In this trial, 3,121 women with operable breast cancer and involved lymph nodes were randomly assigned after surgical treatment to receive cyclophosphamide (600 mg/m2) with doxorubicin (60, 75, or 90 mg/m2) for four cycles, followed by no further therapy or four cycles of paclitaxel (175 mg/m2). Tamoxifen was given to 94% of patients with hormone receptor-positive tumors. No dose effect of doxorubicin was apparent in the trial; disease-free survival at 5 years was 69%, 66%, and 67% in the 60-, 75-, and 90-mg groups, respectively. The addition of paclitaxel was associated with significant hazard reductions of 17% for recurrence (P = .0023) and 18% for death (P = .0064).
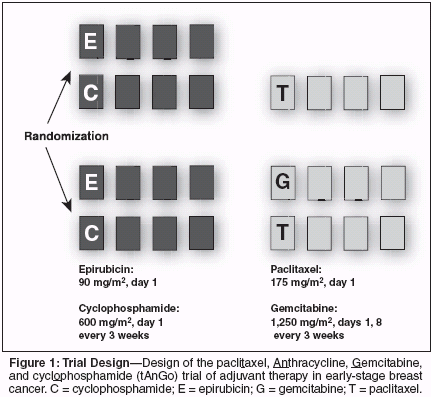
An unplanned, retrospective subset analysis using data from a median follow-up of 30 months indicated no difference in disease-free survival among patients with estrogen receptor (ER)-positive disease when paclitaxel was or was not added to treatment (hazard ratio, 0.92; 95% confidence interval [CI] = 0.73-1.16), and a significant difference among patients with ER-negative disease favoring the paclitaxel-containing regimen (hazard ratio, 0.68; 95% CI = 0.55-0.85). As a result of these findings showing paclitaxel benefit limited to ERnegative disease, the initial tAnGo trial design called for enrollment of patients with ER-negative disease or unknown ER status. The unplanned analysis based on more mature CALGB 9344 data (median follow-up, 69 months) indicated that the addition of paclitaxel was associated with hazard ratios for recurrence of 0.72 (95% CI = 0.59-0.86) in patients with ER-negative tumors and 0.91 (95% CI = 0.78- 1.07) for those with ER-positive tumors, almost all of whom had received adjuvant tamoxifen.[1] The effect of the addition of paclitaxel among patients with ER-negative disease was no longer significant on this analysis after adjustment for multiple comparisons. Several considerations have resulted in modification of THE tAnGo trial eligibility criteria to include enrollment of patients with ER-positive breast cancer, with stratification by ER status. It is a clinical observation that disease events occur earlier in patients with ER-poor disease than in those with ER-positive disease, and it is conceivable that the addition of paclitaxel to standard treatment in the CALGB 9344 trial might show increasing benefit in ER-positive disease with longer follow-up. Indeed, there is some evidence from other trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) study 28 and the Breast Cancer International Research Group (BCIRG) 001 study (which included prospective stratification by hormonereceptor status), that trends in taxane benefit in ER-positive disease emerge over longer-term follow-up.[2,3] Selection of Gemcitabine as Paclitaxel Partner When the tAnGo trial was being designed, capecitabine (Xeloda) and vinorelbine (Navelbine) were the primary candidates for use with a taxane in the study regimen. Capecitabine and vinorelbine both have established roles in the treatment of patients with anthracycline-pretreated breast cancer. In preliminary feasibility studies, however, both agents, when paired with a taxane, exhibited nonhematologic toxicities considered unacceptable for the adjuvant clinic. Capecitabine was associated with liver and skin toxicities. Vinorelbine was very myelosuppressive and was associated with a high incidence of neuropathy, including bilateral cranial nerve problems. Although the role of gemcitabine was less well established at that time, the drug had a number of desirable characteristics supporting its use in the study regimen, including AN absence of cross-resistance with paclitaxel, a nonoverlapping toxicity profile, and high response rates (and complete response rates) when used in combination with paclitaxel in phase II studies in advanced disease. For example, data available at the time included a trial of gemcitabine/paclitaxel in 44 previously treated patients, 93% of whom had received previous anthracycline chemotherapy.[4] The response rate was 45% with the combination, and the complete response rate was 16%.[4] In a study in 43 patients with two or three relapses during anthracycline-based chemotherapy, the gemcitabine/paclitaxel combination produced a response rate of 55%, including a complete response in 17%.[5] In a trial of 43 patients receiving first-line treatment (most of whom had received adjuvant therapy), 68% responded to the combination, including 21% with a complete response.[6] The gemcitabine/paclitaxel combination had also shown promising activity when used with epirubicin in the GET (gemcitabine/epirubicin/paclitaxel) regimen. An overall response rate of 90.2% (complete response, 29.3%; partial response, 61%) was reported by Gennari et al from their multicenter Italian study at the European Breast Cancer Conference in 2004.[7] Of the 41 patients who completed the GET program and underwent surgery, 6 patients (14.6%) had a pathologic complete response, and 3 were downgraded to negative axillary nodes. The gemcitabine/paclitaxel combination was found to be superior to paclitaxel alone in a phase III trial in anthracycline-pretreated patients receiving first-line treatment for metastatic disease (JHQG).[8] On interim analysis, O'Shaughnessy et al initially reported that gemcitabine/paclitaxel was associated with a significantly greater time to disease progression (5.4 vs 3.5 months, P = .0013), with a significantly reduced hazard ratio for disease progression (0.734; 95% CI: 0.607-0.889; P = .0015). Objective response rates were 39.3% with gemcitabine/ paclitaxel and 25.6% with paclitaxel alone (P = .0007). In 2004, Albain et al subsequently presented overall survival data from this study: the overall survival hazard ratio (HR) was 0.775, significantly in favor of the combination (P = .018).[9] They also demonstrated a statistically significant advantage of gemcitabine/ paclitaxel over paclitaxel (18.5 vs 15.8 months; HR = 0.775; P = .018). Oneyear survival was significantly increased for the gemcitabine/paclitaxel arm (70.7% vs 60.9%; P = .019), and the HR favoring gemcitabine/paclitaxel persisted (Cox regresion) after adjusting for baseline covariates: 0.740 (P = .006). Thirty-eight percent of patients in the gemcitabine/paclitaxel arm stopped therapy due to progressive disease, vs 55% for those receiving paclitaxel alone, and therapy ended due to adverse events in 6.7% of patients receiving gemcitabine/ paclitaxel, vs 5.0% of those getting paclitaxel. The tAnGo Trial Design The tAnGo trial is a randomized, open-label, phase III trial evaluating epirubicin/cyclophosphamide followed by paclitaxel alone or paclitaxel/ gemcitabine as adjuvant therapy in early-stage breast cancer.[10] The trial has a target population of 3,000 patients. The coordinating center is the Cancer Research United Kingdom Clinical Trials Unit at The University of Birmingham, United Kingdom. Seventy centers in the United Kingdom are actively enrolling patients, and another 19 have local research ethics committee approvals. Ongoing expansion of the study involves Irish and Spanish collaborative groups and the Central European Cooperative Oncology Group. Key patient eligibility criteria include histologically confirmed earlystage breast cancer; fully resected disease; any nodal status; disease of any ER/progesterone-receptor status; no previous malignancy (unless disease free for at least 10 years); no previous chemotherapy or radiation therapy for breast cancer; and randomization within 8 weeks of surgery. Patients are randomly assigned to receive epirubicin (90 mg/m2 on day 1) and cyclophosphamide (600 mg/m2 on day 1) every 3 weeks for four cycles, followed by either paclitaxel (175 mg/m2 alone on day 1) or paclitaxel (175 mg/m2 on day 1) plus gemcitabine (1,250 mg/m2 on days 1 and 8) every 3 weeks for four cycles (Figure 1). Doses selected for the paclitaxel/ gemcitabine combination are based on phase I studies of the combination in platinum-resistant breast cancer (and solid tumors), wherein essentially full doses of both agents could be combined easily in this setting.[ 11-14] Response rates in the phase I and II studies were in the 45% range, with all dosing schedules examined. The primary end point of the tAn- Go trial is 5-year disease-free survival. Secondary end points include 5- and 10-year overall survival comparisons, 10-year disease-free survival, toxicity, dose intensity, tolerability, and serious adverse events. Prespecified, event-driven interim analyses should permit generation of useful efficacy and safety data shortly after completion of patient accrual. To date, 750 patients have been enrolled, reflecting an initially slow accrual that accelerated markedly in 2003. Of these patients, the first ~600 had ERnegative disease, having been enrolled prior to the protocol change permitting enrollment of patients with any ER status. Approximately half of these 600 patients had disease involving four or more nodes. Other ongoing/ planned substudies include safety, quality-of-life, and tumor marker/pathology parameters. Results of the tAnGo trial, along with results of an ongoing NSABP trial examining adjuvant therapy with paclitaxel/gemcitabine using an every-2-week schedule, should provide important information on the utility of the paclitaxel/gemcitabine combination in the adjuvant setting. Conclusion The randomized, open-label, multicenter phase III tAnGo trial is investigating adjuvant treatment with epirubicin/cyclophosphamide for four cycles followed by paclitaxel alone or combined with gemcitabine for four cycles in patients with early-stage breast cancer. Gemcitabine has been included as a partner for paclitaxel in the tAnGo trial based on high response rates, including high complete response rates, in phase II trials of the combination in more advanced disease and on the safety profile compared with that of other taxane-containing two-drug combinations. The tAnGo trial has a target population of 3,000, and trial data should further elucidate the role of gemcitabine in adjuvant therapy for breast cancer.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Henderson IC, Berry DA, Demetri GD, et al: Improved outcomes from adding sequential paclitaxel but not from escalating doxoru bicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976-983, 2003.
2. Mamounas EP, Bryant J, Lembersky BC, et al: Paclitaxel (T) following doxorubicin/cyclophosphamide (AC) as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28 (abstract 12). Proc Am Soc Clin Oncol 22:4, 2003.
3. Martin M, Pienkowski T, Mackey J, et al: TAC improves DFS and OS over FAC in node positive early breast cancer patients, BCIRG001: 55 months follow-up (abstract 43). Proceedings of the San Antonio Breast Conference; December 2003; San Antonio, Texas.
4. Sánchez-Rovira P, Medina MB, Mohendano N, et al: Results from a phase II study of gemcitabine in combination with paclitaxel in metastatic breast cancer (abstract). Ann Oncol 9(suppl 4):A77P, 1998.
5. Murad AM, Guimaraes RC, Aragao BC, et al: Gemcitabine and paclitaxel as salvage therapy in metastatic breast cancer. Am J Clin Oncol 24:264-269, 2001.
6. Colomer R, Llombart A, Lluch A, et al: Biweekly paclitaxel and gemcitabine in advanced breast cancer: Phase II trial and predictive value of HER2 extracellular domain (abstract 373). Proc Am Soc Clin Oncol 19:97a, 2000.
7. Gennari A, Santoro A, Bottini A, et al: Gemcitabine/epirubicin/paclitaxel (GET) as primary chemotherapy in stage II-IIIA operable breast cancer: Final results of a multicenter Italian study (abstract 64). Proceedings of the European Breast Cancer Conference; 2004.
8. O'Shaughnessy J, Nag S, Calderillo-Ruiz G, et al: Gemcitabine plus paclitaxel (GT) versus paclitaxel (T) as first-line treatment for anthracycline pre-treated metastatic breast cancer (MBC): Interim results of a global phase III study (abstract 25). Proc Am Soc Clin Oncol 22:7, 2003.
9. Albain KS, Nag S, Calderillo-Ruiz G, et al. Global phase III study of gemcitabine plus paclitaxel (GT) vs. paclitaxel (T) as frontline therapy for metastatic breast cancer (MBC): First report of overall survival (abstract 510). Proc Am Soc Clin Oncol 23:5, 2004.
10. Available at http://www.cancerstudies. b h a m . a c . u k / Tr i a l s / Te a m s / B r e a s t / TANGO_Protocol.pdf
11. Poole CJ, Perren T, Hogberg H, et al: Phase I study to investigate the optimal dose and schedule of gemcitabine (G) and paclitaxel (P) in combination in patients with previously treated epithelial ovarian cancer (abstract). Proc Am Soc Clin Oncol 17:A1380, 1998.
12. Rinaldi D, Lormand N, Brierre J, et al: A phase I trial of gemcitabine and paclitaxel in patients with advanced solid tumors, administered every 21 days (LOA-2) (abstract). Proc Am Soc Clin Oncol 17:A848, 2000.
13. Sanchez P, Medina MB, Mohedano A, et al: Results from a phase II study of gemcitabine in combination with paclitaxel in metastatic breast cancer (abstract). Ann Oncol 9(suppl 4):A77P, 1998.
14. Colomer R, Llombart A, Lluch A, et al: Paclitaxel/gemcitabine administered every two weeks in advanced breast cancer: Preliminary results of a phase II trial. Semin Oncol 27(1 suppl 2):20-24, 2000.