Gemcitabine in Combination With Trastuzumab and/or Platinum Salts in Breast Cancer Cells With HER2 Overexpression
Trastuzumab (Herceptin) is an effective treatment in patients withHER2-overexpressing metastatic breast cancer. Risk of trastuzumabinducedcardiotoxicity raises concerns regarding combined use withanthracyclines or other potentially cardiotoxic agents followinganthracycline treatment. We characterized interactions betweentrastuzumab and gemcitabine (Gemzar) and the combination ofgemcitabine and cisplatin or carboplatin (Paraplatin) as such combinationsmight help reduce the risk of cardiotoxicity. Multiple drugeffect/combination index isobologram analysis was used to study theefficacy of chemotherapeutic drug plus trastuzumab combinations inHER2-overexpressing breast cancer cell lines. Combination index valueswere derived from parameters of the median effect plots, and statisticaltests were used to determine whether the mean combinationindex at multiple effect levels significantly differed from a combinationindex value of 1.0 (values < 1.0 indicate synergy; values > 1.0,antagonism; values equal to 1.0, additivity). At a wide range of clinicallyachievable drug concentrations, interactions between trastuzumaband gemcitabine were synergistic at low concentrations of gemcitabineand antagonistic at high concentrations. A consistent synergistic interactionwas observed with the three-drug combination of trastuzumabplus gemcitabine plus carboplatin or cisplatin. Available clinical dataon the use of trastuzumab plus gemcitabine, and trastuzumab plusgemcitabine/paclitaxel, as well as clinical data on the use ofgemcitabine/cisplatin in breast cancer, are discussed. These findingsindicate that trastuzumab plus gemcitabine and trastuzumab plusgemcitabine plus cisplatin or carboplatin are rational combinations toevaluate in clinical trials.
Trastuzumab (Herceptin) is an effective treatment in patients with HER2-overexpressing metastatic breast cancer. Risk of trastuzumabinduced cardiotoxicity raises concerns regarding combined use with anthracyclines or other potentially cardiotoxic agents following anthracycline treatment. We characterized interactions between trastuzumab and gemcitabine (Gemzar) and the combination of gemcitabine and cisplatin or carboplatin (Paraplatin) as such combinations might help reduce the risk of cardiotoxicity. Multiple drug effect/combination index isobologram analysis was used to study the efficacy of chemotherapeutic drug plus trastuzumab combinations in HER2-overexpressing breast cancer cell lines. Combination index values were derived from parameters of the median effect plots, and statistical tests were used to determine whether the mean combination index at multiple effect levels significantly differed from a combination index value of 1.0 (values < 1.0 indicate synergy; values > 1.0, antagonism; values equal to 1.0, additivity). At a wide range of clinically achievable drug concentrations, interactions between trastuzumab and gemcitabine were synergistic at low concentrations of gemcitabine and antagonistic at high concentrations. A consistent synergistic interaction was observed with the three-drug combination of trastuzumab plus gemcitabine plus carboplatin or cisplatin. Available clinical data on the use of trastuzumab plus gemcitabine, and trastuzumab plus gemcitabine/paclitaxel, as well as clinical data on the use of gemcitabine/cisplatin in breast cancer, are discussed. These findings indicate that trastuzumab plus gemcitabine and trastuzumab plus gemcitabine plus cisplatin or carboplatin are rational combinations to evaluate in clinical trials.
The anti-HER2 monoclonal antibody trastuzumab (Herceptin) is active as a single agent in HER2-positive metastatic breast cancer[ 1,2] and effectively prolongs time to disease progression and overall survival in this setting when combined with chemotherapy.[3] Gemcitabine (Gemzar) is a deoxycytidine analog antimetabolite with broad clinical activity against a variety of cancers, including breast cancer.[4,5]
We characterized the in vitro drug interactions between gemcitabine and trastuzumab using the multiple drug effect/combination index method. We further wanted to test whether gemcitabine would increase the synergistic cytotoxic effects of combinations of carboplatin (Paraplatin) or cisplatin with trastuzumab against HER2- overexpressing cell lines. By using the median effect equation of Chou and the combination index equation of Chou and Talalay, quantitation of synergism or antagonism at different concentration and effect levels allows one to select the best pair, or even triplet, of drugs to combine for potentially maximal antitumor efficacy.[ 6,7]
Multiple Drug Effect Analysis in HER2-Overexpressing Breast Cancer Cell Lines
Aliquots of 3 * 103 to 5 * 103 SKBR- 3 and BT-474 cells were plated in 96-well plates. After 24 hours, experimental media containing either an excipient control, trastuzumab, a chemotherapeutic agent, or the combination of trastuzumab plus the chemotherapeutic agent were added to appropriate wells, and serial twofold dilutions were made to span clinically relevant concentration ranges. These concentrations were sufficient to inhibit growth of control cells by 20% to 90% [IC20 to IC90]. For the analysis of drug interac- tions between gemcitabine and platinum salts, either an excipient control, chemotherapeutic agents, or the combination of both chemotherapeutic agents was added to appropriate wells, and serial twofold dilutions were made to span clinically relevant concentration ranges.
Following cell incubation, for 72 to 120 hours, the media was removed, the plates were washed with phosphate- buffered saline (PBS), and the cells were stained with 0.5% N-hexamethylpararosaniline (crystal violet) in methanol. To each well, 0.1 mL of Sorenson's buffer (0.025M sodium citrate, 0.025M citric acid in 50% EtOH) was added to solubilize the stain, and the plates were analyzed in an enzyme-linked immunosorbent assay (ELISA) plate reader at 540-nm wavelength. Absorbance at this wavelength is proportional to the number of cells.[8,9] All assays were performed in triplicate.
The results are expressed as percent cell growth relative to growth of control cells. For each assay, the log of the fractional growth inhibition was plotted against the log of the drug concentration, and the Pearson correlation coefficient (r) from the linear regression curve was calculated. To ensure quality control, all r values were required to be > .85 for the data to be subjected to multiple drug effect analysis. Multiple drug effect analysis was performed using computer software from Biosoft (Cambridge, UK) as described; details of this methodology have been published previously.[ 6,7,10]
Interaction Between Gemcitabine and Trastuzumab
The interaction between the combination of gemcitabine and trastuzumab was concentration dependent, ranging from synergistic at low concentrations of gemcitabine (eg, ≤ 106 nmol) to additive or antagonistic at high concentrations of gemcitabine (≥ 212 nmol; IC80 to IC90; Figure 1). The mean combination index values were 1.44 (95% confidence interval [CI] = .56 to 2.32; P = .311) in SKBR- 3 cells (Figure 1A), and 0.71 (95% CI = 0.43 to 0.99; P = .039) in BT- 474 cells (Figure 1B). However, the mean combination index values do not precisely reflect the concentration dependence of the gemcitabine interactions. The concentration dependence of the in vitro interaction between trastuzumab and gemcitabine makes it difficult to predict the nature of the interaction between the two drugs in vivo, where the drug concentrations vary according to their pharmacokinetic characteristics and intratumoral distribution.
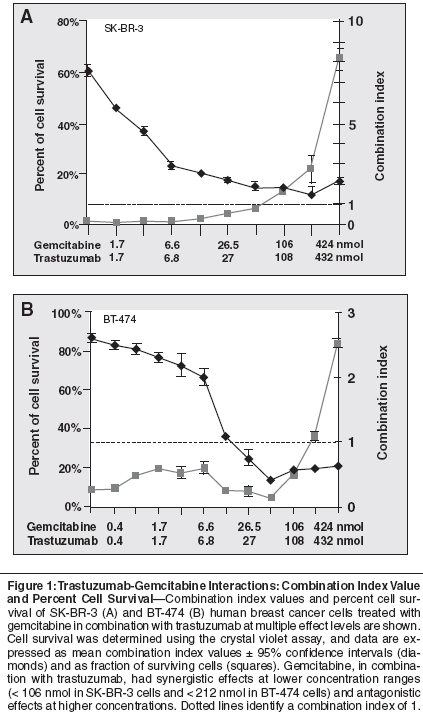
Interaction Between Gemcitabine and Cisplatin or Carboplatin
Earlier reports have shown that the nature of interactions between cisplatin and gemcitabine were synergistic in lung cancer cell lines and suggested the potential for greater efficacy with this combination than with either agent alone.[11] Furthermore, these findings are consistent with the results of clinical studies that have investigated the combination of cisplatin and gemcitabine.[ 12,13]
Because the synergy between gemcitabine and cisplatin has only been reported for lung cancer cell lines and has not yet been described in breast cancer cells, it was important to investigate whether gemcitabine acts synergistically with both cisplatin and carboplatin in HER2-overexpressing breast cancer cell lines as well (Figure 2).
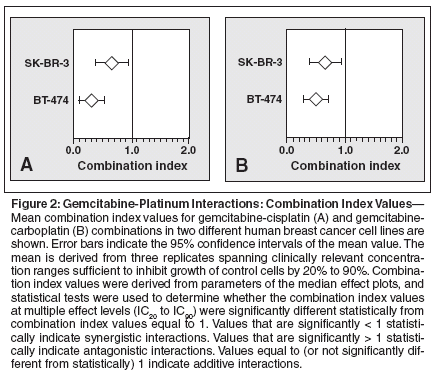
When gemcitabine was combined with cisplatin, the mean combination index values were 0.73 (95% CI = 0.57-0.89; P = .001) in SK-BR-3 cells and 0.36 (95% CI = 0.26-0.45; P < .001) in BT-474 cells, indicating a synergistic interaction in both cell lines (Figure 2A). When gemcitabine was combined with carboplatin, the mean combination index values were 0.76 (95% CI = 0.58-0.94; P = .006) in SKBR- 3 cells, 0.56 (95% CI = 0.41- 0.71; P < .001) in BT-474 cells, confirming a synergistic interaction in both cell lines (Figure 2B).
Interaction Between Gemcitabine, Cisplatin or Carboplatin, and Trastuzumab
We previously reported synergistic interactions between cisplatin or carboplatin and the anti-HER2 monoclonal antibodies 4D5 and trastuzumab. Combination index values were consistently < 1.0 for all cell lines tested.[10,14,15]
Having observed that gemcitabine, cisplatin, and carboplatin, when combined with trastuzumab, have synergistic cytotoxic effects against HER2-overexpressing cell lines, we wanted to determine whether combinations of these agents would further increase cytotoxicity. Therefore, we investigated the combination of gemcitabine, cisplatin or carboplatin, and trastuzumab using the multiple drug effect/combination index model. This combination yielded strong synergistic interactions across a wide concentration range of clinically relevant doses (Figure 3). The mean combination index value for this three-drug combination was 0.63 (95% CI = 0.50-0.76; P < .001) in SK-BR-3 cells and 0.54 (95% CI = 0.39-0.69; P < .001) in BT-474 cells when using cisplatin (Figure 3A). The mean combination index value for this three-drug combination was 0.63 (95% CI = 0.51 to 0.75; P < .001) in SK-BR-3 cells and 0.74 (95% CI = 0.63 to 0.85; P < .001) in BT-474 cells when using carboplatin (Figure 3B), suggesting that both drug combinations are highly synergistic in vitro.
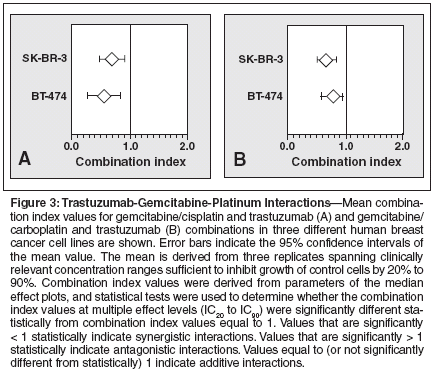
Clinical Effects of the Trastuzumab/Gemcitabine Combination
The effects of combination treatment with trastuzumab and gemcitabine were evaluated in a phase II trial in heavily pretreated patients with HER2-positive metastatic breast cancer.[ 16,17] In this trial, 59 patients with HER2 2+ or 3+ overexpression on immunohistochemistry (IHC) were treated with gemcitabine at 1,200 mg/m2 on days 1 and 8 every 21 days plus trastuzumab as a 4-mg/kg loading dose and 2 mg/kg weekly. In total, 88% of patients had received adjuvant therapy, and 84% and 52% had received previous chemotherapy with anthracyclines and taxanes, respectively. Patients had received one (23%), two (45%), or ≥ three (27%) previous courses of chemotherapy for metastatic disease. Twenty-one patients had HER2 2+ disease and 38 had HER2 3+ disease.
The objective response rate after trastuzumab/gemcitabine treatment was 37%; 24% of patients with HER2 2+ disease and 45% of those with HER2 3+ disease responded. Overall, the median time to disease progression was 5.8 months, with a median overall survival of 14.7 months. Grade 3/4 toxicities were infrequent, consisting of neutropenia (15%/3%), asthenia (4%/0%), fever (3%/1%), dyspnea (6%/0%), abdominal/back pain (2%/1%), edema (1%/0%), and nausea (1%/0%). Preliminary safety analysis indicated that there was an absence of clinically significant cardiac toxicity with this regimen; four patients experienced reduced leftventricular ejection fractions that did not require cessation of study treatment.
Miller and colleagues with the Hoosier Oncology Group reported preliminary findings of another phase II trial that indicated clinical antitumor activity of the triple combination of trastuzumab at a loading dose of 4 mg/kg followed by 2 mg/kg weekly, plus gemcitabine at 1,200 mg/m2 on days 1 and 8, and paclitaxel at 175 mg/m2 on day 1, every 21 days. Patients had not received previous chemotherapy for metastatic disease or previous gemcitabine or trastuzumab treatment.[18,19] Results showed an objective response rate of 67% (12/18) and a median time to treatment failure of 9 months.
With the addition of paclitaxel, hematologic toxicity was more frequent than with the trastuzumab/gemcitabine regimen. Grade 3/4 neutropenia occurred in 62%/36% with thrombocytopenia in 15%/2% of patients. Severe nonhematologic toxicities were infrequent. Clinical congestive heart failure was observed in three patients, including two who entered treatment with left-ventricular ejection fractions of approximately 50% and became symptomatic with small additional reductions; treatment was discontinued in the three patients, who subsequently recovered.
Clinical Effects of Gemcitabine/ Cisplatin Combinations in Metastatic Breast Cancer
Heinemann reviewed the combination of gemcitabine and cisplatin and noted that it is an effective combination as first-line chemotherapy for patients with metastatic breast cancer. Gemcitabine/cisplatin achieved a response rate of 80% (25/31) in one phase II trial,[20] and other studies in intensively pretreated breast cancer patients demonstrated a median response rate of 43% (range: 26%- 50%).[15] Moreover, the toxicity profile was moderate, with thrombocytopenia and neutropenia being reported as the main side effects in these studies. The gemcitabine/cisplatin combination offers a tolerable and effective treatment option, particularly for patients with disease progression after treatment with anthracyclines and/or taxanes.
Moreover, as previously discussed, in that review, and elsewhere in this volume, based on preclinical data, the rationale to combine platinum agents with gemcitabine might lie in their ability to elicit complementary activity. For example, the platinum agents cisplatin and carboplatin increase the activity of DNA repair mechanisms, and gemcitabine inhibits DNA repair mechanisms (eg, masked-chain termination). Thus, tumor cells that are exposed to cisplatin or carboplatin are uniquely sensitive to gemcitabine.
Conclusion
The combination of trastuzumab and gemcitabine is active in HER2- positive metastatic breast cancer. Given the concern over augmented cardiotoxicity with combined use of anthracyclines and trastuzumab, the gemcitabine/trastuzumab combination is an attractive option as first-line treatment and alternative in patients who may be at increased risk of trastuzumab- associated cardiotoxicity due to previous anthracycline treatment. The clinical activity of gemcitabine, carboplatin, cisplatin, and trastuzumab as single agents against breast cancer is well established.[ 2,4,21]. Therefore, the combination of trastuzumab and gemcitabine plus cisplatin or carboplatin, given the magnitude of observed in vitro synergy among these agents, in addition to the potential absence of an anthracycline antibiotic in this combination, may be potentially useful for clinical evaluation.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article
References:
1. Cobleigh MA, Vogel CL, Tripathy D, et al: Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2- overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17:2639-2648, 1999.
2. Vogel CL, Cobleigh M, Tripathy D, et al: First-line, single-agent Herceptin (trastuzumab) in metastatic breast cancer: A preliminary report. Eur J Cancer 37:25-29, 2001.
3. Slamon DJ, Leyland-Jones B, Shak S, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001.
4. Carmichael J, Possinger K, Phillip P, et al: Advanced breast cancer: A phase II trial with gemcitabine. J Clin Oncol 13:2731-2736, 1995.
5. Carmichael J: The role of gemcitabine in the treatment of other tumours. Br J Cancer 78(suppl 3):21-25, 1998.
6. Chou T, Talalay P: Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enz Regul 22:27-55, 1984.
7. Chou TC, Motzer RJ, Tong Y, et al: Computerized quantitation of synergism and antagonism of Taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: A rational approach to clinical protocol design. J Natl Cancer Inst 86:1517-1524, 1994.
8. Flick DA, Gifford GE: Pharmacokinetics of murine tumor necrosis factor. J Immunopharmacol 8:89-97, 1986.
9. Reile H, Birnbock H, Bernhardt G, et al: Computerized determination of growth kinetic curves and doubling times from cells in microculture. Anal Biochem 187:262- 267, 1990.
10. Pegram M, Hsu S, Lewis G, et al: Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18:2241-2251, 1999.
11. Bergman AM, Ruiz van Haperen VW, Veerman G, et al: Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 2:521-530, 1996.
12. Gridelli C, Gallo C, Shepherd FA, et al: Gemcitabine plus vinorelbine compared with cisplatin plus vinorelbine or cisplatin plus gemcitabine for advanced non-small-cell lung cancer: A phase III trial of the Italian GEMVIN Investigators and the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 21:3025-3034, 2003.
13. Heinemann V: Gemcitabine plus cisplatin for the treatment of metastatic breast cancer. Clin Breast Cancer 3(suppl 1):24-29, 2002.
14. Pietras RJ, Pegram MD, Finn RS, et al: Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 17:2235-2249, 1998.
15. Pegram MD, Konecny GE, O'Callaghan C, et al: Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96:739-749, 2004.
16. O'Shaughnessy JA, Vukelja S, Marsland, T, et al: Gemcitabine and trastuzumab for HER- 2 positive metastatic breast cancer: Preliminary results of a phase II study (abstract 523). Breast Cancer Res Treat 69:302, 2001.
17. O'Shaughnessy J: Gemcitabine and trastuzumab in metastatic breast cancer. Semin Oncol 30(2 suppl 3):22-26, 2003.
18. Miller KD, Sisk J, Gize G, et al: Phase II study of gemcitabine, paclitaxel and trastuzumab in metastatic breast cancer. A Hoosier Oncology Group trial. Program and abstracts of the 25th San Antonio Breast Cancer Symposium; December 11-14, 2002; San Antonio, Texas. Breast Cancer Res Treat 70:A437, 2002.
19. Sledge GW Jr: Gemcitabine, paclitaxel, and trastuzumab in metastatic breast cancer. Oncology 17(suppl 14):33-35, 2003.
20. Ruiz GC, Fuentes de la Pena, Meza FA, et al: A phase II study of gemcitabine plus cisplatin in metastasic breast cancer (abstract 1988). Proc Am Soc Clin Oncol 20:2001.
21. Martin M, Diaz-Rubio E, Casado A, et al: Carboplatin: An active drug in metastatic breast cancer. J Clin Oncol 10:433-437, 1992.