Adjuvant Therapy in Gastric Cancer: Can We Prevent Recurrences?
Despite a dramatic decline in the incidence of gastric carcinoma inthe United States during the past century, treatment remains a challengingproblem for oncologists. Surgery continues to be the primarymodality for managing early-stage gastric cancer, but up to 80% ofpatients who undergo a "curative" resection develop locoregional ordistant recurrence. Given these sobering statistics, there has been greatinterest in developing strategies to prevent recurrences after surgeryand improve overall mortality. In this article, we review data onadjuvant treatment modalities for this disease, including radiotherapy,chemotherapy, combination chemotherapy and radiation, intraperitonealtreatment, and immunotherapy. We focus attention on the recentwidespread acceptance of adjuvant chemoradiotherapy, based on theresults of Intergroup trial 0116. Future strategies incorporating differentmodalities of treatment will be outlined.
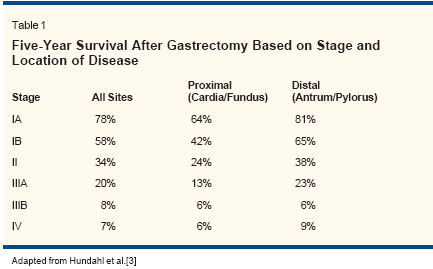
ABSTRACT: Despite a dramatic decline in the incidence of gastric carcinoma inthe United States during the past century, treatment remains a challengingproblem for oncologists. Surgery continues to be the primarymodality for managing early-stage gastric cancer, but up to 80% ofpatients who undergo a "curative" resection develop locoregional ordistant recurrence. Given these sobering statistics, there has been greatinterest in developing strategies to prevent recurrences after surgeryand improve overall mortality. In this article, we review data onadjuvant treatment modalities for this disease, including radiotherapy,chemotherapy, combination chemotherapy and radiation, intraperitonealtreatment, and immunotherapy. We focus attention on the recentwidespread acceptance of adjuvant chemoradiotherapy, based on theresults of Intergroup trial 0116. Future strategies incorporating differentmodalities of treatment will be outlined.In 2003, an estimated 22,400 individualsin the United States willbe diagnosed with gastric cancer,and 12,100 will die from the disease.[1] The worldwide incidence isapproximately 550,000 cases peryear, with 405,000 deaths.[2] Despiteattempts at curative resection, survivalfor this disease remains poor(Table 1).[3] As a result, investigatorshave been evaluating adjuvanttherapy to prevent recurrences andimprove on overall mortality.Studies of the benefits of adjuvanttherapy for gastric adenocarcinomahave yielded conflicting results. Themore favorable results reported inAsian countries compared to Westerncountries may relate to ethnicityfactors,[4] although differences intumor location, prevalence of earlystagedisease, and extent of preoperativestaging evaluation may alsoaccount for some of these results.[5]Such discrepancies make it difficultto translate the results of studies inone of these populations to the other.Treatment OptionsAdjuvant and NeoadjuvantRadiotherapy
Gastric cancer has generally beenconsidered a relatively radioresistantcarcinoma, with limited benefits insymptom palliation for advanced disease.[6] However, the high incidenceof locoregional recurrence after surgeryfor early disease has sparkedinterest in radiation as adjuvant orneoadjuvant therapy. Among patientswho undergo a curative resection,38% to 67% will develop a clinicallyevident locoregional recurrence, withnodal stage and number of positivelymph nodes predictive of risk.[7]Investigators have hypothesized thatradiotherapy may sterilize the localbed and prevent the growth of presumedresidual microscopic diseaseafter surgery.Few trials have studied the impactof radiation alone as adjuvant therapy.The British Stomach CancerGroup randomized 436 patients whohad undergone curative resection tono further therapy, adjuvant radiotherapy,or adjuvant chemotherapy.The 5-year overall survival for the153 patients randomized to adjuvantradiotherapy was 12%, compared to20% for those assigned to surgeryalone (P > .2).[8]In China, researchers randomized370 patients with locally advancedgastric cancer of the cardia to eitherimmediate surgery or preoperativeradiation (40 Gy) followed by surgery.[9] Patients who received neoadjuvantradiation had a significantlyimproved 5-year overall survival(30% vs 20%, P = .009) and a lowerlocal recurrence rate (39% vs 52%,P < .025) compared to those whowere treated with surgery alone. Althoughthese results are intriguing,the authors did not provide informationon preoperative staging, and thestudy was limited to patients withcardiac and gastroesophageal junctioncancers.Recently, Skoropad et al reportedon a trial of preoperative radiotherapywith 20 years of follow-up.[10]Of 152 patients who underwent exploratorylaporatomy for staging, 102were randomized to a short course ofpreoperative therapy (20 Gy over 5days) followed by surgery, or surgeryalone. The authors did not findany significant differences in survivalbetween the two treatment groups(P = .56). Moreover, subset analysesby tumor location or stage of diseasedid not demonstrate any significantsurvival differences between treatmentarms. Further studies with radiationalone are unlikely, given therenewed interest in the combinationof chemotherapy and radiotherapy inthe adjuvant setting.Intraoperative Radiotherapy
Intraoperative radiotherapy (IORT)may be an attractive option forachieving local control in gastric cancerdue to its ability to deliver a single,large fraction of radiation withminimal damage to normal tissue.[9]Although most studies of IORT havebeen nonrandomized and conductedby single institutions, several experiencesare notable.In Japan, a retrospective case-controlcomparison in 242 patients demonstratedthat those with stage II-IVdisease who received IORT achieveda 10% to 20% improvement in 5-yearsurvival.[11] In contrast, Sindelar andcolleagues at the National Cancer Institute(NCI) randomized 41 gastriccancer patients to either externalbeamradiation or IORT after surgery.[12] Although locoregionalfailure was less common among patientsin the IORT arm (44% vs 92%,P < .001), overall survival did notdiffer between treatment arms. A randomizedtrial in Germany comparedsurgery alone to surgery with IORTand also found no statistical differencesin overall survival or cancerrelateddeath.[13]Systemic Therapy
Approaches to adjuvant chemotherapyfor gastric cancer have beenbased largely on the results of regimensevaluated in the setting of metastaticdisease. Unfortunately, the useof chemotherapy in patients with advancedgastric cancer has produceddisappointing results. Completeresponses are rare, and partial responsesto single-agent therapies havebeen limited, with results rangingfrom 0% to 30%.[14] Fluorouracil(5-FU) remains the most widely usedagent, with response rates to singleagenttherapy in previously untreatedpatients ranging from 21% to30%.[15,16] Other agents with activityas single-agent therapy includecisplatin, doxorubicin, epirubicin(Ellence), docetaxel (Taxotere), paclitaxel,and irinotecan (CPT-11,Camptosar).[14]Combination chemotherapy regimensfor metastatic gastric cancerhave been tested extensively. Initialtrials have often demonstrated strikingresults, with the majority of patientsachieving a response. However,confirmatory trials in larger populationsof patients have resulted in moresobering findings.[17] Many combinationregimens have not beenshown to improve overall survivalcompared to single-agent therapywhen tested in prospective, randomizedtrials.[18-21]Recently, the combination of epirubicin,cisplatin, and 5-FU (ECF) producedimproved response and overallsurvival rates with lower toxicity,compared to a prior standard of care,FAMTX (5-FU, doxorubicin [Adriamycin],methotrexate).[22] However,the median overall survival amongpatients treated with ECF was still only8.7 months, with a 2-year survival rateof 14%. Other promising combinationregimens include irinotecan or a taxaneand a platinum agent,[14] but randomized,multicenter trials must beconducted to discern the true efficacyof these newer drugs in gastric adenocarcinomas.Adjuvant Chemotherapy
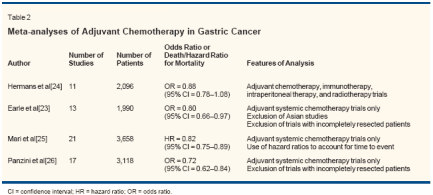
The administration of chemotherapyshortly after complete resectionof gastric tumors in patients at highrisk of recurrence has been assessedas a means of treating clinically undetectablemicrometastases. A numberof studies have investigatedwhether adjuvant chemotherapy canreduce the incidence of the diseaserecurrence and mortality followinga curative resection. To date, four meta-analyses have been publishedthat examined the impact of postoperativeadjuvant chemotherapy onsurvival after surgical resection(Table 2).[23-26]
Hermans et al analyzed 11 randomizedtrials, with a total of over2,000 patients, that compared postoperativechemotherapy to surgeryalone.[24] Most of these trials usedolder 5-FU-based regimens, includingFAM (5-FU/doxorubicin/mitomycin[Mutamycin]), 5-FU/mitomycin,and 5-FU/semustine (methyl-CCNU).The investigators were not able todemonstrate a significant survivaladvantage for adjuvant chemotherapybeyond that achieved with surgeryalone. However, the analysis waslater criticized for a lack of sufficientstatistical power and the studies chosenfor inclusion.Earle and Maroun compiled asimilar meta-analysis, limited to non-Asian studies and with stricter inclusioncriteria.[23] The authorsreported an odds ratio for death inthe treated group of 0.80 (95% confidenceinterval [CI] = 0.66-0.97), correspondingto a relative risk of 0.94(95% CI = 0.89-1.00). They calculatedthat in a group of patients similarto those included in the analyzedtrials, 65% would have a recurrenceand die of gastric cancer followingtreatment with surgery alone, comparedto 61% following treatmentwith surgery and adjuvant chemotherapy.This absolute risk reductionof 4% indicates that 25 patients wouldneed to receive adjuvant therapy toprevent one death (ie, the "numberneeded to treat" = 25).An Italian group led by Mari performeda meta-analysis of 20 trialspublished between 1982 and 1999.[25]Using time-to-event data with censoring,they found a favorable hazard ratioof 0.82 (95% CI = 0.75-0.89) forpostoperative adjuvant chemotherapyover surgery alone. In a subgroup analysis,they did not detect any statisticaldifferences between trials, with orwithout the use of anthracycline-basedtherapy. Most recently, Panzini andcolleagues reported a meta-analysissimilar to that of Mari et al, althoughtrials that did not require complete resectionof disease were excluded.[26]Of the 17 trials included, 14 overlappedwith those in the analysisby Mari et al. The odds ratio fordeath for patients receiving adjuvanttherapy in this analysis was 0.72(95% CI = 0.62-0.94).Despite the modestly positive resultsof more recent meta-analyses,enthusiasm for adjuvant systemic chemotherapyremains limited. Theseanalyses are tempered by the usual restraints,including publication bias,differences in patient populations andentry criteria of the trials, and a nonrandomizeddesign. Indeed, no individual,prospective randomized trialhas provided convincing evidence infavor of adjuvant chemotherapy. Inaddition, the authors of each of thesefour meta-analyses argue that larger,randomized trials are warranted andthat adjuvant chemotherapy after curativeresection for gastric cancershould be considered investigational.Neoadjuvant Chemotherapy
The use of neoadjuvant chemotherapyto downstage locally advanceddisease and improve the resectabilityof the primary tumor in gastric cancerpatients has attracted the attentionof researchers. Phase I/II studies havedemonstrated the feasibility of preoperativechemotherapy, with downstagingachieved in up to 50% ofpatients and complete pathologicresponses in up to 10%.[27-30] In addition,operative morbidity and mortalitywere not increased with the useof preoperative therapy. However,prospective randomized trials havenot demonstrated a benefit for neoadjuvantchemotherapy.The Dutch Gastric Cancer Grouprandomized 56 patients to preoperativeFAMTX or immediate surgery.[31] In this small trial, 44% ofpatients did not complete the plannedfour cycles of FAMTX due to pro-
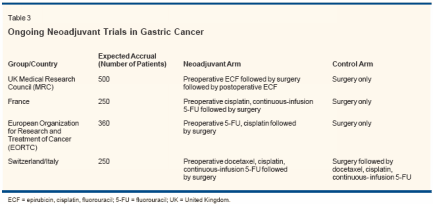
gressive disease, and a nonsignificantimprovement in resection rates wasseen in the surgery-only arm. Moreover,patients randomized to preoperativeFAMTX did not experienceany survival benefit compared to thosereceiving surgery alone. Preliminaryreports of neoadjuvant chemotherapytrials conducted in Asia have alsofailed to demonstrate improved survivalrates for preoperative therapy.[32,33] Several large, randomizedtrials are currently being conductedin Europe to evaluate the role of neoadjuvantchemotherapy in the treatmentof gastric cancer (Table 3).[14]Adjuvant Chemoradiotherapy
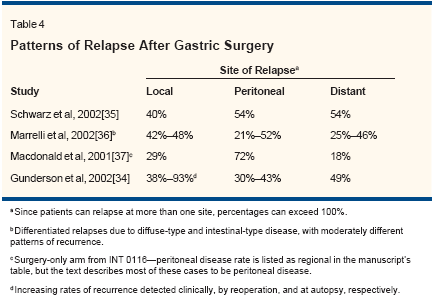
Gastric adenocarcinoma has a tendencyto recur both locoregionallyand distantly following curative resection(Table 4).[34-37] As a result,many investigators have explored thecombination of chemotherapy andradiotherapy to prevent tumor recurrence.Several phase II trials havedemonstrated favorable long-termsurvival with the use of chemoradiotherapyafter curative resection, with5-year survival rates ranging from21% to 55%.[38]Previous randomized trials comparingsurgery alone to chemoradiotherapyafter surgery, however, werefraught with design flaws and did not
convincingly support adjuvant treatment.[39,40] Dent et al randomized142 patients with all stages of gastriccancer, including T4 and M1 disease,to surgery alone or postoperativetherapy including 2,000 cGy ofradiotherapy delivered in eight fractionsand chemotherapy with either5-FU or thiotepa.[39] No differencesin survival rates (P > .5) or quality oflife emerged between the control andtreatment arms. Of note, the radiationand chemotherapy doses in thistrial would now be considered suboptimal.Subsequently, Moertel and colleaguesrandomized 62 patients withresectable gastric cancer to surgeryalone or postoperative radiotherapywith 3,750 cGy plus 5-FU.[40] Dueto a later-realized design flaw, informedconsent was obtained afterrandomization and ~25% of patientsin the postoperative therapy arm re-
fused treatment. Intent-to-treat analysisdemonstrated an improvementin 5-year survival in the adjuvanttherapy arm (23% vs 4%, P < .05).However, when the treatment groupwas subdivided according to thosewho actually received treatment andthose who refused it, 5-year survivalrates between these subgroups andthe control group were not statisticallydifferent. Of note, treatmentdid reduce locoregional recurrences(39% for treated patients comparedto 54% for those who received notreatment). The authors concluded,"this study does not establish 5-FUplus radiation as effective surgicaladjuvant therapy for gastric cancerbut suggests this approach as apossible fruitful area for continuedresearch."[40]
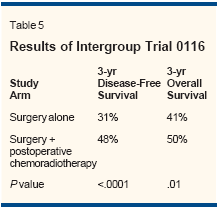
- INT 0116-Ultimately, between1991 and 1998, a large, US intergroupadjuvant chemotherapy andradiation therapy trial (INT 0116)was initiated by the Southwest OncologyGroup (SWOG).[37] Investigatorsrandomized 556 patients withresected stage IB-IV, M0 gastric andgastroesophageal adenocarcinoma toeither no further therapy or postoperativechemoradiotherapy to investigatethe potential benefit ofadjuvant chemoradiotherapy. Patientswere stratified by tumor stageand nodal status. Those randomizedto chemoradiotherapy received onecourse of 5-FU at 425 mg/m2/d andleucovorin at 20 mg/m2/d on days 1through 5, followed by 45 Gy ofradiation (to the original tumor bed,regional lymph nodes, proximal anddistal resection margins plus 2 cm)concurrently with bolus 5-FU at 400mg/m2 and leucovorin at 20 mg/m2on each of the first 4 and last 3 daysof radiotherapy. Two additional cyclesof 5-FU and leucovorin wereadministered 1 month after radiationtherapy.A single radiation protocol coordinatorreviewed all radiotherapytreatment plans. Of note, more thanone-third of the initial plans wererevised upon review. Patients treatedwith postoperative chemoradiationachieved a significant improvementin both disease-free and overall survivalat 3 years (Table 5). Theseresults translated into a 52% improvementin relapse-free survival and a35% improvement in overall survivalfor patients in the intervention arm,after adjustment for extent of invasionthrough the stomach wall andnodal status.
- INT 0116 Limitations-Despitethese encouraging findings, the studyhas generated several criticisms.[41]First, many patients underwent a suboptimalexamination for lymph nodemetastases; more than one-half (54%)had a D0 dissection (fewer than sevenlymph nodes identified in the surgicalspecimen), whereas only 10%had a D2 dissection (extended lymphadenectomy).However, there is nodefinitive evidence that a D2 dissectionoffers any survival advantageover a less radical resection.[42] Furthermore,rates of D0, D1, and D2dissections in the intergroup trial arecomparable to those in general practice,suggesting that the findings canbe generalized to the population atlarge.[3]In addition, INT 0116 includedpatients with a wide variety of pathologicstages. This criticism questionsthe benefit of and need for adjuvanttherapy in patients with the less-representedextreme stages of disease,particularly T1 and N0. Much of thebenefit of chemoradiation in this trialappears to relate to improved localcontrol rather than prevention of distantdisease, and although patientstreated with postoperative chemoradiotherapyexperienced fewerlocoregional recurrences, distant recurrenceswere equivalent betweenthe two arms. Finally, postoperativeadjuvant chemoradiotherapy aftergastrectomy can be difficult to administerin full; in INT 0116, only65% of patients in the treatment armcompleted all prescribed therapy.
- Subsequent Research-Based onthe results of INT 0116, curativeintentsurgery followed by adjuvantchemoradiotherapy has become thestandard of care for gastric cancer inNorth America. However, because thebenefits associated with postoperativechemoradiotherapy were principallylimited to improvements inlocoregional control, investigatorshave examined the use of systemicregimens that are potentially moreactive than 5-FU/leucovorin.
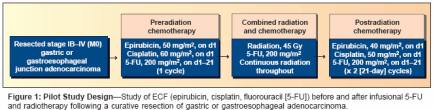
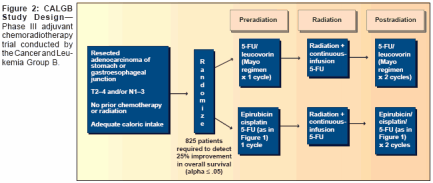
- Fuchs and colleagues conducted amulticenter, pilot study of ECF pluscontinuous-infusion 5-FU administeredduring external-beam irradiation(Figure 1).[43] Among 21patients receiving therapy, the toxicityprofile was tolerable and comparableto that of INT 0116. At a medianfollow-up of 8.1 months (range:1-27 months), only 14% of patientsexperienced a documented relapse.Because this pilot study demonstratedthat postoperative ECF and continuous-infusion 5-FU with radiationwas well tolerated, an NCI-sponsoredtrial was initiated that will randomize825 patients to this regimen orthe INT 0116 regimen after a curativeresection of gastric or gastroesophagealadenocarcinoma (Figure 2).In a similar effort, the RadiationTherapy Oncology Group (RTOG) isconducting a randomized, phase IItrial of two cisplatin- and paclitaxelcontainingregimens for adjuvanttreatment of gastric or gastroesophagealcancer. Patients with stage IB-IIIB cancer will be randomly assignedto one of two groups. Patients ingroup 1 will receive a 5-day continuousinfusion of 5-FU and a 1-hourinfusion of cisplatin once a day for5 days in weeks 1 and 5. They willalso receive a 24-hour continuous infusionof paclitaxel during weeks 1and 5. At least 3 weeks later, patientswill undergo radiation therapy 5 daysper week and will receive a 5-daycontinuous infusion of 5-FU and a3-hour infusion of paclitaxel once perweek for 5 weeks.Patients in group 2 will receive a3-hour infusion of paclitaxel and a1-hour infusion of cisplatin in weeks1 and 5. Between 3 and 6 weekslater, they will undergo radiation therapyplus a 96-hour continuous infusionof paclitaxel and a 1-hourinfusion of cisplatin once per weekfor 5 weeks. The planned accrual ofthis trial is 94 patients. The results ofthis trial will help investigators determinewhich chemoradiation regimenis sufficiently efficacious andtolerable to warrant a phase III comparisonwith the INT 0116 regimen.
Intraperitoneal Therapy
The high frequency of peritonealrecurrences after surgical resectionhas generated considerable interestin intraperitoneal therapy. In oneAsian trial, Hagiwara et al randomized50 patients with resectable T3/4gastric cancer to either surgery aloneor surgery followed by intraperitonealtherapy. Intraperitoneal therapyconsisted of perioperative delayedreleasemitomycin adsorbed ontoactivated charcoal (M-CH).[44] Patientsrandomized to intraperitonealM-CH experienced a significant benefit in overall survival comparedto those who were randomized tosurgery alone.
As a result, a follow-up study conductedin Europe randomized a similarpatient population to eithersurgery plus M-CH or surgery alone.The trial was closed prematurely dueto an increased rate of postoperativecomplications and mortality in theintraperitoneal therapy arm.[45] Similarly,Yu et al randomized 248 patientswith all stages of gastric cancerto surgery alone or surgery withperioperative intraperitoneal mitomycinand 5-FU.[46] Overall, intraperitonealtherapy provided nosurvival benefit, although a subsetanalysis demonstrated a moderateimprovement in survival amongstage III patients.Other small randomized trials usingeither intraperitoneal 5-FU, mitomycin,or cisplatin have also failedto show a significant benefit for intraperitonealtherapy.[47] Consequently,the value of postoperativeintraperitoneal therapy in resectedgastric cancer remains uncertain.An alternative approach to intraperitonealtherapy is continuous hyperthermicperitoneal perfusion(CHPP). With such a strategy, eithermitomycin or cisplatin is administeredfor 1 to 2 hours after primarytumor resection during laparatomyin patients with peritoneal disease orthose considered at high risk of recurrence.[48] Small randomizedstudies of CHPP have been reportedfrom Japan; three studies demonstrateda statistically significant survivaladvantage, whereas two did not showa significant difference among patientstreated perioperatively withCHPP vs those treated with surgeryalone.[49-52] Again, larger studieswill be necessary to establish the efficacyof this therapy.Several groups have conductedcombined-modality studies incorporatingpostoperative intraperitonealtherapy with systemic chemotherapy.Leichman et al assigned 38 patientswith resectable gastric tumorsto preoperative chemotherapy withinfusional 5-FU, leucovorin, and cisplatinfollowed by surgery, intraperitonealfloxuridine, and cisplatin.[53]At a median follow-up of 19 months,only 14% of patients developed arecurrence, and the median survivalwas at least 17 months.Similarly, Kelsen and colleaguestreated 56 high-risk gastric cancerpatients with three cycles of FAMTXfollowed by surgery; in addition, patientsreceived postoperative intraperitoneal5-FU and cisplatin andintravenous 5-FU.[54] Of the 89% ofpatients who underwent surgery, 61%had potentially curative resections.The median survival for all patientswas 15.3 months; however, only 16%had documented peritoneal recurrences.Although the integration of intraperitonealtherapy into other adjuvantstrategies is attractive, further studieswill be necessary to isolate theimpact of intraperitoneal delivery ofchemotherapy on survival, diseaserecurrence, and quality-of-life endpoints.
Immunotherapy
Investigators have studied the impactof immune-modulating agentson disease recurrence and overall survival.Among the most commonlystudied agents are a protein-boundpolysaccharide extracted from themycelia of the mushroom
Coriolusversicolor
(polysaccharide-K, krestin)and the streptococcal lysateOK-432 (available as Picibanil inJapan).Studies randomizing patients tosystemic chemotherapy plus immunotherapyor systemic therapy alonehave produced conflicting results(reviewed by Toge[55]). Sakamotoet al, in a recent meta-analysis of 13trials comparing the addition ofOK-432 to conventional chemotherapyvs chemotherapy alone in curativelyresected gastric cancerpatients, found no survival benefitfor the addition of immunotherapy(
P
= .27).[56] Similarly, preoperativeintratumoral injection ofOK-432 did not provide a significantsurvival advantage compared tosurgery alone.[57]
Future Strategies
Beyond standard cytotoxic chemotherapies,future studies will likelyexamine more targeted therapiesagainst a variety of specific molecularmarkers overexpressed in tumorcells. Studies have demonstratedoverexpression of epidermal growthfactor, epidermal growth factorreceptor, matrix metalloproteinase,c-Met, cyclooxygenase-2, andc-erbB-2; microsatellite instability;and alterations in p53, fragile histidinetriad, trefoil factor family 1, andbeta-catenin in gastric tumors (reviewedby El-Rifai and Powell).[58]Although the utility of these findingsto individual patients may not be immediatelyclear, an understanding ofgastric cancer at the molecular levelmay allow for rational therapeuticdecisions, as well as novel, more targetedtreatments in the future. Potentiallyless toxic biologic therapies areespecially attractive in the adjuvantsetting.
Conclusions
Gastric adenocarcinoma remainsa difficult problem for the oncologycommunity. Although the majorityof patients with stage IA disease arecured with surgical resection, fewerthan 15% of patients in the UnitedStates are diagnosed at that stage.[3]Patients are often diagnosed with locallyadvanced disease, and despiteattempts at curative resection, themajority will develop tumor recurrence.INT 0116 has established adjuvant5-FU, leucovorin, andradiotherapy as the new standard ofcare after a curative gastrectomy.However, given the modest benefitassociated with this treatment, futuregenerations of clinical trials will needto incorporate more rational, targetedtherapies into the adjuvant treatmentof gastric adenocarcinoma.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.