Adjuvant Treatment of Breast Cancer in the Elderly
Breast cancer is the most common life-threatening malignancy in women, and the second leading cancer killer of women, claiming the lives of over 40,000 American women annually. Breast cancer incidence increases with advancing age until age 80, and the median age at diagnosis is 61.
ABSTRACT: ABSTRACT: Breast cancer is the most common malignancy in women, and the incidence of breast cancer increases with increasing age until age 80. As the population continues to age, breast cancer in older women will become an increasing clinical challenge. A majority of breast cancer in older women is hormone receptorâpositive, making hormonal therapy the mainstay of adjuvant treatment in this group. Chemotherapy clinical trials have largely excluded the elderly, and therefore limited data exists on the efficacy and tolerability of standard adjuvant chemotherapy in older women. Ongoing studies are addressing alternatives to standard adjuvant chemotherapy in this group. Emerging biologic instruments such as gene-expression profiling may be able to better identify patients who will gain benefit from adjuvant chemotherapy. Elderly patients are highly variable in their functional status, reserve capacity, and comorbidity, and better assessment tools are needed to evaluate these issues and predict which patients will tolerate cancer treatments. More work is needed to address the many challenges that affect the adjuvant treatment of breast cancer in older women.
Breast cancer is the most common life-threatening malignancy in women, and the second leading cancer killer of women, claiming the lives of over 40,000 American women annually.[1] Breast cancer incidence increases with advancing age until age 80, and the median age at diagnosis is 61.[2] The population, in America and worldwide, is aging due to improvements in health care and nutrition, and it is estimated that the number of Americans over the age of 65 will increase from 30 million in 2000 to over 71 million in 2030.[3] Given the relationship between age and breast cancer incidence, as the absolute number of women over age 65 (and even over 85) grows, breast cancer will be an increasingly common diagnosis.
More specifically, breast cancer in older women will become even more prevalent, and we must improve our understanding of how best to treat these women. This will require clinical trials focusing specifically on treatments in this age group and an improved understanding of the complex interactions between cancer biology, cancer therapy, normal physiologic changes of aging, comorbid illness, and functional status. The goal of such research should be to develop treatments that reduce cancer recurrence and mortality while having minimal detrimental effect on quality of life and comorbidity.
Adjuvant Therapy Background
The breast cancer mortality rate fell 24% between the years 1990 and 2000 for women aged 30 to 79 years.[4] This improvement in breast cancer mortality resulted from both early detection through screening and advancements in adjuvant treatment of early-stage disease. Depending on the model of risk reduction, adjuvant therapy has been estimated to be responsible for 35% to 72% of that reduction.[5] Adjuvant treatment of breast cancer is designed to treat micrometastatic disease or breast cancer cells that have escaped the breast and regional lymph nodes but have not yet established an identifiable metastasis. Treatment at this point is aimed at reducing the risk of future recurrence, thereby reducing breast cancer–related morbidity and mortality.
The time required for a micrometastasis to develop into clinically relevant metastatic disease is variable and relates to the growth rate and invasive characteristics of the primary tumor. For example, high-grade, estrogen receptor (ER)-negative, HER2-positive breast cancer is most likely to recur within the first 2 years following adjuvant therapy. Low-grade ER-positive breast cancers, on the other hand, are known to recur at a relatively constant rate for up to 25 years after primary therapy. Adjuvant treatment, then, is only effective if two conditions are met: (1) the patient's competing causes of mortality allow her to live long enough to see the reduction in recurrence, and (2) the treatment itself doesn't result in toxicities that are worse than the cancer recurrence it prevented. Therefore, it is paramount to factor in the treatment's potential to reduce risk against the patients' breast cancer–independent life expectancy and the potential toxicity of the therapy.
Lack of Data on the Elderly
Unfortunately, very few adjuvant studies have focused specifically on elderly patients, and even subset analyses of existing trials looking at elderly participants are difficult to perform, as the elderly constitute a small minority of subjects in cancer clinical trials. The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) performed a meta-analysis of 194 randomized trials of adjuvant chemotherapy or hormonal therapy performed between 1985 and 2000, and presented the data on the benefits of treatment.[6] However, even in this meta-analysis, there were so few patients over the age of 70 in the chemotherapy trials that no reliable information could be extracted on the benefit of chemotherapy in that age group. Similarly, in a review of Southwest Oncology Group studies in breast cancer, only 9% of participants were over the age of 65, while 49% of women diagnosed with breast cancer in the United States during that time were over age 65.[7]
Therefore, treatment recommendations for older patients currently must be extrapolated from data obtained in a younger cohort. Fortunately, this is being increasingly recognized, and efforts are underway to obtain more evidence-based guidelines for the treatment of older breast cancer patients. Despite the lack of clear guidelines, older women should still be considered candidates for adjuvant treatment of breast cancer. The average life expectancy for average-health 70- and 80-year-old women is still 16 and 9 years, respectively,[8] and reasonable efforts should be made to minimize the effect that breast cancer will have on this life expectancy.
Adjuvant Hormonal Therapy
Breast cancer in older women is largely hormone receptor–positive, and therefore, hormonal therapy plays the main role in adjuvant treatment. In two large reviews of breast cancer pathology in elderly women, ER positivity increased with advancing age. For example, in a review of over 3,200 breast tumors from Europe and 800 breast tumors from the Massachusetts General Hospital, ER expression-a known biomarker of improved prognosis-correlated positively with age, with 40% positivity in patients aged 40, 60% positivity in patients aged 60, and over 70% positivity in patients aged 80.[9] Another review of over 50,000 breast tumors in the San Antonio breast cancer databases, and over 250,000 breast tumors in the Surveillance Epidemiology and End Results (SEER) registry, similar results were found. ER positivity was seen in 83% of women aged 55 to 64, 87% of women aged 65 to 74, 90% of women aged 75 to 84, and 91% of women aged 85 and over.[10]
Available Agents
US Food and Drug Administration (FDA)-approved endocrine therapies for the adjuvant treatment of breast cancer include tamoxifen and the aromatase inhibitors (anastrozole [Arimidex], letrozole [Femara], exemestane [Aromasin]). Tamoxifen is a selective estrogen-receptor modulator (SERM) that binds to and inhibits the estrogen receptor in the breast. It has ER-stimulating effects in other tissues, including bone (resulting in bone strengthening) and endometrium (leading to an increased risk of endometrial cancer). Other adverse effects associated with tamoxifen are hot flashes and an increased risk of venous thromboembolism and stroke, similar to that observed with hormone-replacement therapy.[11]
Thromboembolic complications increase with increasing age. A relative risk model derived from published data on adjuvant tamoxifen indicates that tamoxifen use results in an increased risk of mortality from thromboembolism of 1.3 deaths/1,000 women for those age 50, 3.3 for those age 60, 7.5 for those age 70, and 15 for those age 80.[12] Therefore, particular attention must be paid to other risk factors for thromboembolism when considering the use of tamoxifen in older women. Tamoxifen appears to have a beneficial effect on lipids, decreasing total cholesterol by 10% to 15%.[11,13]
Advances in our understanding of tamoxifen metabolism have identified several key factors affecting the conversion of tamoxifen to its active metabolites endoxifen and 4-hydroxytamoxifen. Specifically, polymorphisms in the CYP2D6 genotype have been associated with impaired endoxifen production and poorer clinical outcomes in tamoxifen-treated breast cancer patients.[14] Importantly, the selective serotonin-reuptake inhibitor (SSRI) antidepressant drugs are known to impair CYP2D6 activity (paroxetine and fluoxetine having the most potent inhibitory effects), even in those with a normal genotype, and concomitant use of these agents has also been associated with inferior outcome in women receiving tamoxifen as adjuvant treatment of breast cancer.[15] Therefore, SSRIs should not be used in patients receiving tamoxifen for breast cancer.
Less well described are interactions between other medications and tamoxifen metabolism. For example, one study suggests that diuretics and anti-inflammatory agents (both drugs used commonly in older women) significantly affect tamoxifen metabolism.[16] Continued research in this area will ensure that concomitant medications are not negating tamoxifen's beneficial effect in breast cancer patients.
Aromatase inhibitors function by inhibiting aromatase, the enzyme (found in fat and adrenal glands as well as tumor cells) responsible for converting other steroid hormones into estrogen. Aromatase is the sole source of estrogen in postmenopausal women, and likely the underlying reason that obesity has been associated with a higher risk of breast cancer in postmenopausal patients (ie, a larger volume of body fat produces more estrogen). Adverse effects associated with aromatase inhibitors include arthralgias (seen in up to half of patients in some series), decreased bone density with increased risk of fracture, and a possibly detrimental effect on the lipid profile, although this has not been clearly confirmed in further trials.[11,13,17]
Bone health is particularly important in the elderly population, in whom decreased bone mineral density is common, independent of breast cancer treatment. The bone loss seen with anastrozole in the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial was significantly associated with lower baseline bone mineral density and lower baseline estradiol levels.[18] There appeared to be no independent association with age, but ATAC only enrolled patients up to age 70. Interestingly, bone mineral loss was greater in those within 4 years of menopause, as compared to those more than 4 years postmenopause, suggesting that the older patients are not more susceptible unless they have lower baseline bone mineral density or estradiol levels.
The IV bisphosphonate zoledronic acid (Zometa, Reclast) has been shown to prevent bone loss associated with aromatase inhibitor therapy, even when it is given only once every 6 months.[19] Importantly, no osteonecrosis of the jaw was seen in this study. Thus, lipid monitoring and osteoporosis screening are recommended for all patients on aromatase inhibitors, and the use of statin or bisphosphonate therapy is encouraged to minimize complications.
There are currently no specific recommendations for the treatment of aromatase inhibitor–associated arthralgias. Standard analgesics such as nonsteroidal anti-inflammatory drugs do not seem to be particularly beneficial, but other treatments such as acupuncture are being investigated.
Clinical Trials
The development of hormonal therapy for early-stage ER-positive breast cancer has resulted in dramatic reductions in the risk of recurrence and mortality. Tamoxifen, which has been approved for breast cancer treatment since 1977, has been shown in multiple studies to decrease breast cancer–associated mortality and recurrence.[20] In an analysis of 55 trials evaluating tamoxifen vs placebo in the adjuvant treatment of breast cancer, 5 years of tamoxifen therapy resulted in a 47% reduction in recurrence and a 22% reduction in mortality.[20] The EBCTCG meta-analysis demonstrates that the risk reduction from adjuvant tamoxifen is similar (or even superior) in older vs younger women,[6] with a relative risk of recurrence of 0.49 in those over 70, 0.55 in those 60 to 69, 0.66 in those 50 to 59, and 0.71 in those 40 to 49.
Similar results were seen in a study of 689 women aged 65 to 96 (average age = 74) diagnosed with early-stage breast cancer between 1997 and 1999.[21] The hazard ratio (HR) for breast cancer mortality for receiving tamoxifen vs never receiving tamoxifen in this group was 0.61. More recently, the aromatase inhibitors have shown superiority to tamoxifen in postmenopausal women with metastatic and early-stage breast cancer.
Aromatase inhibitors have been tested in adjuvant clinical trials in comparison to tamoxifen as well as in sequencing trials after 2 or 5 years of tamoxifen use. Results from the ATAC trial showed anastrozole to be superior to tamoxifen in improving disease-free survival (DFS, HR = 0.87), and time to recurrence (HR = 0.79), and decreasing contralateral breast cancer (HR = 0.58),[17] but none of these trials have yielded an improvement in overall survival (OS) compared to tamoxifen. Subgroup analysis of patients over age 65 in the Breast International Group (BIG) 1-98 trial, which compared 5 years of letrozole vs tamoxifen, revealed a hazard ratio for DFS of 0.82, but P = .06, likely due to inadequate numbers.[22] The overall hazard ratio for DFS for all enrolled patients was also 0.82, but with a statistically significant P = .007.
Switching trials, in which aromatase inhibitors are initiated after 2 to 3 years of tamoxifen, have shown improved DFS as well as OS compared to continuing tamoxifen alone.[23] Trials initiating aromatase inhibitors after 5 years of tamoxifen also reveal improved DFS and OS in node-positive patients.[24,25] The optimal duration and sequence for aromatase inhibitors has not been clearly defined, but their benefits in terms of breast cancer recurrence and survival support their use in postmenopausal women, and the benefits seem to be equivalent in older and younger women.
Adjuvant Chemotherapy
While hormonal therapy plays the main role in adjuvant therapy for older women with breast cancer, cytotoxic chemotherapy may also be indicated in certain situations, such as hormone receptor–negative disease, HER2-positive disease, or high-risk hormone receptor–positive disease. The use of chemotherapy in these groups, however, is complicated by the patients' comorbidity, functional status, and individual preference. In addition, clinical research aimed specifically at these issues has been sparse, and therefore clear guidelines for the use of chemotherapy in elderly breast cancer patients have not been developed.
ER-Negative Breast Cancer
In ER-negative breast cancer, for which hormonal therapy will offer no benefit, chemotherapy is the only systemic option for reducing the risk of recurrence. Because ER-negative disease is relatively rare in elderly populations, no randomized data exist on the benefit of chemotherapy. However, in a review of SEER data from 1992 to 1999, women aged 66 and older with ER-negative breast cancer were identified[26]; 34% of the 5,081 women received chemotherapy for their breast cancer. Data were analyzed using multivariate analysis and propensity score methods to minimize selection bias, and using either method, chemotherapy was associated with a statistically significant 15% reduction in mortality. Increased age and higher comorbidity were associated with a lower likelihood of receiving chemotherapy. Tumor size, number of positive nodes, and higher tumor grade were associated with a higher likelihood of receiving chemotherapy. While this was not a randomized study, it does suggest that older women derive significant benefit from systemic chemotherapy for ER-negative breast cancer. Therefore, elderly women with ER-negative breast cancer and a life expectancy of 5 years or more should be offered adjuvant chemotherapy.
Optimal combinations of drugs have not been defined for this group. Potential toxicities of anthracycline vs non–anthracycline-containing regimens must be weighed. Emerging data suggest that anthracycline regimens are superior to nonanthracycline regimens such as CMF (cyclophosphamide, methotrexate, fluorouracil) in HER2-positive patients,[27] possibly in association with topoisomerase II–overexpressing/amplified tumors. As topoisomerase II testing becomes more accurate and widely available, this may provide further guidance in the selection of adjuvant chemotherapy regimens.
In a review of the Cancer and Leukemia Group B (CALGB) experience in adjuvant chemotherapy studies for node-positive breast cancer from 1985 to 1999 (all ages), ER-negative breast cancer patients received the greatest benefit from the following advances in chemotherapy dosing and schedule: (1) increased dose intensity, as in CALGB 8541, (2) the addition of paclitaxel to anthracycline-containing therapy, as in CALGB 9344, (3) the use of dose-dense, or every-2-week chemotherapy administration as in CALGB 9741.[28] All of these advances will require study in elderly cohorts to confirm safety and efficacy in that group.
ER-Positive Breast Cancer
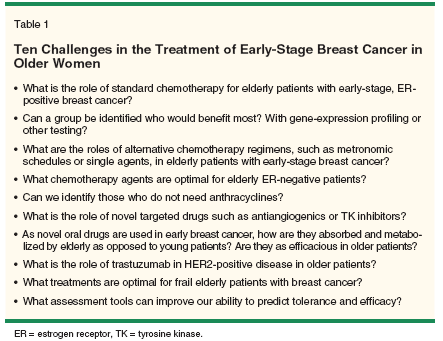
One of the most difficult challenges in the adjuvant treatment of breast cancer is the decision to recommend for or against chemotherapy in hormone receptor–positive disease (Table 1). This is particularly relevant to older breast cancer patients, as a great majority in this age group have ER-positive disease. In the setting of ER-positive breast cancer in any age group, adjuvant hormonal therapy provides the greatest reduction in risk of recurrence, and the additional benefit conveyed by chemotherapy depends upon the other prognostic features and the overall risk.
For example, in the EBCTCG meta-analysis, treatment with tamoxifen provided a 31% proportional reduction in the annual breast cancer mortality rate, for patients of all ages with ER-positive tumors.[17] The addition of anthracycline chemotherapy to tamoxifen provided an additional 26% proportional reduction for women under age 50, and an additional 14% proportional reduction for women aged 50 to 69. These reductions were greatest for patients with node-positive disease. As described above, data were inadequate to make comments about the benefit in women over age 70.
While chemotherapy has been shown to provide an overall benefit in women with ER-positive breast cancer, the benefit is relatively small, and therefore not all women who receive chemotherapy will benefit. Improved mechanisms to identify those most likely to benefit from chemotherapy will allow us to avoid giving chemotherapy to those who would do equally well with hormonal therapy alone. This is particularly important in elderly women, in whom avoiding unnecessary chemotherapy might prevent significant toxicity.
Predictive Tools
• Gene-Expression Profiling-The advent of gene-expression profiling has shed some light on this challenge, and more gene-expression systems are under development. Again, these systems will need to be studied specifically in elderly patients to ensure that the results are applicable to all age groups. The most widely studied gene-expression profiling tool to date is Oncotype DX, a 21-gene assay that evaluates 16 breast cancer–associated genes and 5 reference genes. The breast cancer–associated genes are involved in estrogen signaling, proliferation, HER2 signaling, and invasion. The assay was validated on tumor specimens from patients previously treated on the National Surgical Adjuvant Breast and Bowel Project (NSABP) study B-14, in which women with ER-positive, node-negative breast cancer were treated with tamoxifen or a placebo.[29]
The Oncotype DX assay results in a recurrence score (RS) between 0 and 100. The scores are divided into low (RS < 18), intermediate (RS 18–30), and high (RS > 30) risk of recurrence, and these scores predicted the 10-year risk of relapse in tamoxifen-treated patients. The assay was then applied to patients' specimens from a subsequent NSABP study (B-20), in which women with ER-positive, node-negative breast cancer were randomized to chemotherapy plus tamoxifen or tamoxifen alone.[30] In the overall analysis of this study, the addition of chemotherapy was found to provide a 4% to 5%, statistically significant improvement in distant relapse–free survival (DRFS).
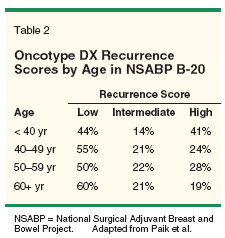
The question then is whether the specific group of patients who benefit can be identified, and whether the RS was able to do so. For patients with a low RS, chemotherapy provided no benefit over tamoxifen alone. For those with a high RS, the benefit from the addition of chemotherapy was large, with a 28% absolute reduction in 10-year DRFS (P = .001). While age was not found to be a predictive factor, a high recurrence score was found in 41% of patients under age 40, 24% of patients 40 to 49, 28% of patients 50 to 59, and only 19% of patients over 60 (Table 2).[30] This indicates that among women with ER-positive, node-negative breast cancer, while fewer elderly patients will benefit from the addition of chemotherapy compared to their younger counterparts, a percentage of women in the elderly group will derive significant benefit.
There are no published data on further age-related variation in RS among age groups over 60. The intermediate RS was seen in a small number of patients, making it difficult to draw conclusions about the benefit of chemotherapy in this group. A study is ongoing (the Trial Assigning IndividuaLized Options for Treatment [Rx], or TAILORx) to better define the benefit in the intermediate RS group. This study randomizes patients with ER-positive node-negative breast cancer and an intermediate RS to hormonal therapy alone or hormonal therapy plus chemotherapy.
In addition to the data on Oncotype DX in node-negative breast cancer, recent analysis of data from the Southwest Oncology Group (SWOG) 8814 trial (which evaluated the addition of anthracycline-containing chemotherapy to tamoxifen in ER-positive, node-positive breast cancer), was presented at the San Antonio Breast Cancer Symposium in December 2007.[31] This analysis revealed that the Oncotype DX assay was predictive of chemotherapy benefit in node-positive patients as well, with subjects who have a low RS gaining no benefit from the addition of chemotherapy to tamoxifen. However, this cohort still had an event rate of 40% at 10 years, suggesting that novel therapies are needed to improve the outcome.
Other gene-expression analysis systems are also under development, including the 70-gene array, MammaPrint, and the 76-gene array, Rotterdam Signature. These both require fresh frozen tissue, which makes it more difficult to apply to many surgical specimens, as compared to Oncotype DX, which is performed on formalin-fixed paraffin-imbedded tissue, as is obtained from any surgical pathology evaluation. These gene-expression profiles and future directions for this method of prognostication and prediction have been reviewed in detail by Henry and Hayes.[32]
• ER Positivity and Age-As stated, few studies have evaluated the benefit of chemotherapy specifically in elderly women. One study by the French Adjuvant Study Group randomized 338 women over age 65 with operable node-positive breast cancer to tamoxifen alone vs tamoxifen plus epirubicin (Ellence).[33] Epirubicin was given at a 30-mg flat IV dose on days 1, 8, and 15 on a 28-day cycle, for six cycles. Tamoxifen was given concurrently with chemotherapy at 30 mg orally daily. Approximately 10% of the patients were ER-negative. Overall, the epirubicin arm had a 3.3% improvement in 6-year DFS (P = .14), as well as a 4.6% reduction in local recurrence (P = .05). However, subset analysis by ER positivity revealed that the DFS benefit was primarily in the ER-negative subset, with a 30% improvement in DFS in that small group. The ER-positive group had only a 1.9% improvement in DFS.
Similarly disappointing results were seen in a review of the efficacy of CMF chemotherapy in addition to tamoxifen in postmenopausal women, when stratified by age.[34] For the women over age 65, the addition of CMF resulted in only a 2% improvement in DFS, while that improvement was 8% for those under 65. However, the test for heterogeneity of CMF effect according to age was not statistically significant. The reduced effectiveness in the older group was not attributed to dose reductions.
Therefore, there is currently little evidence supporting the use of adjuvant chemotherapy in ER-positive elderly breast cancer patients. However, as the Oncotype DX results suggest, there may be a small percentage of elderly women who would benefit from the addition of chemotherapy, and that benefit is obscured by the remainder, who are not gaining benefit from chemotherapy. Perhaps chemotherapy trials targeting elderly women with a high RS would reveal significant benefit in that group.
• Alternative Regimens-Until such data are available, we believe that elderly women with high-risk ER-positive breast cancer who are otherwise healthy should still be considered candidates for chemotherapy and should be informed of the treatment options. Many of these women still relapse and die of advanced breast cancer despite adjuvant hormonal therapy, and therefore further research is needed to identify additional treatments to reduce the relapse and mortality from breast cancer in this group. Alternatives to standard adjuvant chemotherapy regimens are being considered, such as the addition of bisphosphonates to hormonal therapy, metronomic schedules of chemotherapy, or the addition of different chemotherapy agents and targeted agents to hormonal therapy.
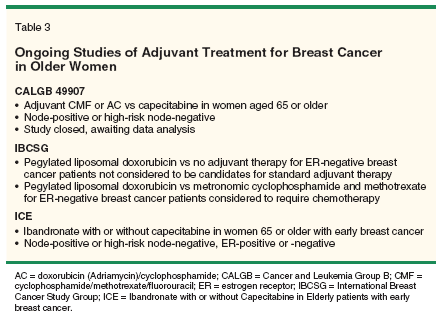
For example, the CALGB recently completed a study comparing 6 months of oral capecitabine (Xeloda) vs standard adjuvant chemotherapy in women over age 65 with early-stage breast cancer (Table 3). Results of this study are anxiously awaited. In addition, the International Breast Cancer Study Group (IBCSG) is investigating pegylated liposomal doxorubicin (Doxil) vs no therapy as well as pegylated liposomal doxorubicin vs a low-dose metronomic schedule of oral cyclophosphamide and oral methotrexate in elderly women who are not considered candidates for standard chemotherapy.
A European Intergroup study, the Ibandronate with or without Capecitabine in Elderly patients (ICE) trial, is evaluating the use of ibandronate (Boniva, an oral bisphosphonate used for osteoporosis that might also inhibit bone metastasis) with or without capecitabine in elderly node-positive or high-risk node-negative patients. In addition, as metronomic dosing schedules become better understood in breast cancer in general, these low-dose continuous schedules will need to be evaluated in older women specifically.
All of this research is being conducted in the hope of identifying novel regimens that will be better tolerated yet still offer risk reduction in elderly patients with high-risk localized disease. Perhaps the use of a predictive tool such as Oncotype DX will be incorporated into future studies to better select women most likely to benefit from additional treatment.
• Role of Trastuzumab-Another important issue in the treatment of elderly breast cancer patients is the unclear role of trastuzumab (Herceptin), with or without concurrent chemotherapy, in the adjuvant treatment of HER2-positive disease. HER2-positive breast cancer is known to portend a more aggressive biology, with increased risk of relapse and mortality. Multiple studies have demonstrated a significant reduction in relapse and mortality with the addition of trastuzumab to standard chemotherapy in early-stage disease. A meta-analysis of the five randomized controlled trials comparing chemotherapy to chemotherapy plus trastuzumab revealed an overall odds ratio of mortality of 0.52 (P < .0001), and an overall odds ratio for recurrence of 0.53 (P < .00001).[35] However, few elderly were treated in these trials, with median ages ranging from 49 to 51.
The HERA trial randomized HER2-positive patients to trastuzumab (1 or 2 years) or observation following completion of standard adjuvant or neoadjuvant chemotherapy.[36] Over 5,000 women were randomized, and only 16% of participants were over age 60. Following an editorial request for information on the elderly participants, the HERA authors stated that the study was not powered for subgroup analysis.[37] However, in the participants over age 60, 14.3% of the patients in the trastuzumab-treated group experienced an event (defined as breast cancer recurrence, new breast cancer, second nonbreast malignancy, or death from any cause), compared to 15% of the women over age 60 in the control arm. This is in comparison to a 13% event rate among all patients treated with trastuzumab. Therefore, it appears that among older patients, trastuzumab provided a slight advantage, but this was not statistically significant and not as prominent as the benefit seen in younger women, perhaps due to the small number of older patients or due to higher rates of other events such as death from a competing cause of mortality.
In addition, grade 3 or 4 adverse events were seen in 14.7% of the over-60 trastuzumab-treated patients compared to 11% of the total population of trastuzumab-treated patients. Symptomatic heart failure was seen in 3% of the older trastuzumab recipients compared to 2% of all trastuzumab-treated patients. While the authors suggest that these limited data should not prevent older patients from receiving adjuvant trastuzumab, they do agree that more information is needed to clarify the efficacy and safety of trastuzumab in this population. None of the other adjuvant trastuzumab studies have published data on the efficacy by age group.
HER2 overexpression, as currently defined, is relatively uncommon in older women, and is often associated with concurrent ER positivity. Future challenges for HER2-positive early breast cancer in older women include the need to define the role of adjuvant trastuzumab with alternative chemotherapy regimens such as single-agent paclitaxel, define the role of adjuvant trastuzumab and hormonal therapy without cytotoxic chemotherapy, evaluate alternative populations who might benefit from anti-HER2 therapy such as those with polysomy for HER2, and define the role of lapatinib (Tykerb) in the adjuvant treatment of breast cancer, as it may produce less cardiotoxicity than trastuzumab.
Chemotherapy Toxicity in the Elderly
While healthy elderly individuals generally tolerate chemotherapy as well as younger patients,[38] there are a few specific toxicities which are more common in the older patient. Myelosuppression is the main dose-limiting toxicity for many cytotoxic chemotherapies in the adjuvant and metastatic setting, and is an especially difficult problem in the elderly. Even healthy elderly have decreased bone marrow cellularity and dysregulated cytokine production, leading to increased sensitivity of the marrow to chemotherapy. Multiple studies have revealed that myelosuppression and the risk of febrile neutropenia are nearly doubled in elderly patients receiving aggressive chemotherapy.[39,40] In a study of elderly breast cancer patients, those receiving an anthracycline-based therapy were at the highest risk.[41]
The National Comprehensive Cancer Network (NCCN) guidelines[42] have recommended the prophylactic use of hematopoietic growth factors such as granulocyte colony-stimulating factor (G-CSF, Neupogen) in elderly patients receiving any moderately myelosuppressive chemotherapy, as opposed to their recommendations for nonelderly patients, in whom growth factor is used prophylactically only with high-risk regimens. Aggressive prophylactic use of growth factor in elderly patients has been shown to decrease the risk of febrile neutropenia and improve quality of life and cost of care, but has not shown a mortality benefit to date.[43]
In addition to myelosuppression, chemotherapy (with anthracyclines in particular) is associated with cardiotoxicity and a risk of acute myeloid leukemia (AML). A review of the SEER database evaluated women aged 66 to 80 with no history of congestive heart failure (CHF) diagnosed with stage I–III breast cancer, and assessed the risk of cardiotoxicity according to the type of adjuvant treatment used.[44] The adjusted hazard ratio for CHF was 1.26 for women aged 66 to 70 receiving anthracycline-containing regimens compared with other chemotherapy. This increased risk of CHF in the anthracycline-treated group increased over time. Interestingly, there was no difference in the risk of CHF for women aged 71 to 80. The authors suspect that selection bias for treatment type in this group may have been higher, total anthracycline dose may have been lower, or that the higher overall risks of CHF in older age groups may have obscured any treatment-related difference in this group. In this study, other baseline characteristics associated with an increased risk of CHF included black race, trastuzumab therapy, hypertension, diabetes, and coronary artery disease. Left-sided radiation therapy was not associated with an increased risk.
Adjuvant Radiation Therapy
Standard local therapy for early breast cancer involves mastectomy or breast-conserving therapy followed by radiation therapy. In addition, radiation is used in patients undergoing mastectomy if the tumor is large or multiple nodes are involved. A number of investigators are evaluating whether the standard radiation recommendations are applicable to older patients. For example, the CALGB performed a study of patients aged 70 or older with clinical stage I, ER-positive breast cancer; patients were randomized to receive tamoxifen alone or radiation therapy and tamoxifen.[46] The addition of radiation resulted in a small decrease in the rate of local relapse (4% for tamoxifen only vs 1% for tamoxifen plus radiation), but there was no difference in rates of mastectomy for local recurrence, distant metastasis, or 5-year survival. Further studies are needed to clearly define which patients may safely be treated without radiation.
Comorbidity and Geriatric Assessment
Regardless of the potential risk reduction with various therapies, the individual patient's comorbidities and ability to tolerate such treatments must be assessed and taken into consideration when planning adjuvant therapy. Clearly, functional age is highly variable; some 80-year-olds have the functional status and reserve capacity of an average 60-year-old, and vice versa. Therefore, improved mechanisms to reliably predict how patients will tolerate therapy are desperately needed.
Various forms of comprehensive geriatric assessment have been used by geriatricians for years to assess the many domains of elder life. These include comorbidity, nutrition, social support, polypharmacy, and cognition, and such assessments can identify previously unrecognized problems that may complicate therapy.[47,48] These issues are obviously important to a patient's ability to undergo complicated cancer treatments requiring frequent clinic visits, multiple medications, and multiple potential side effects. The elderly cancer patient must be able to juggle all of these issues in the face of her underlying problems, which might include difficulty with transportation, malnutrition, or memory loss. Therefore, several geriatric assessments for oncology are under development to provide quick tools for identifying key problems that the oncologist needs to address, in order to safely and effectively treat the patient.[49]
Conclusions
Breast cancer in older women will become an increasingly common diagnosis as the population continues to age. Many challenges remain in the treatment of these patients. Many have hormone receptor–positive disease, and hormonal therapy is a mainstay of treatment in these women. Chemotherapy provides a clear benefit in healthy older women with ER-negative disease. However, the benefits of chemotherapy in ER-positive disease are unclear, and much work is needed to answer a number of important questions: (1) What is the role of standard adjuvant chemotherapy in this group? (2) What is the role of alternative adjuvant chemotherapy regimens such as metronomic schedules or single agents? (3) What is the role of other drugs such as bisphosphonates or novel targeted antineoplastics? (4) What options are there for frail patients? (5) How can we better predict tolerance and efficacy for any of these regimens? (6) Can predictive tools such as gene-expression profiles identify older women most likely to benefit from treatments? Further, the role of trastuzumab, with or without chemotherapy or hormonal therapy, must be better defined in older patients. Increasing attention to these important issues will result in focused clinical trials, which will allow the development of evidence-based recommendations for this growing group of breast cancer patients.
This article is reviewed here.
References:
References
1. National Cancer Institute: Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review, 1975-2002, based on SEER data submission 2004. Available at http://seer.cancer.gov/csr/1975_2002/. Accessed Feb 5, 2008.
2. American Cancer Society: Breast Cancer Facts & Figures 2007-2008. Atlanta, American Cancer Society, 2007.
3. US Census Bureau: US Interim Projections by Age, Sex, Race, Hispanic Origin, 2004. Available at www.census.gov/ipc//www/usinerimproj/. Accessed Nov 20th 2007.
4. Ries L, Eisner M, Kosary C, et al: SEER cancer statistics review, 1975-2001. Bethesda, Md; National Cancer Institute; 2004.
5. Berry DA, Kronin KA, Plevritis SK, et al: Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784-1792, 2005.
6. Early Breast Cancer Trialists’ Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of randomised trials. Lancet 365:1687-1717, 2005.
7. Hutchins LF, Unger LM, Crowley JJ, et al: Underrepresentation of patients 65 years of age or older in cancer treatment trials. N Engl J Med 341:2061-2067, 1999.
8. Arias E: United States life tables, 2002. Natl Vital Stat Rep 53:1-38, 2004.
9. Eppenberger-Castori S, Moore DH Jr, Thor AD, et al: Age-associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol 34:1318-1330, 2002.
10. Diab SG, Elledge RM, Clark GM: Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92:550-556, 2000.
11. Buzdar A, Howell A, Cuzick J, et al, for the Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group: Comprehensive side effect profile of anastrazole and tamoxifen as adjuvant treatment for early stage breast cancer: Long term safety analysis of the ATAC trial. Lancet Oncology 7:633-643, 2006.
12. Ragaz J, Coldman A: Survival impact of adjuvant tamoxifen on competing causes of mortality in breast cancer survivors, with analysis of contralateral breast cancer, cardiovascular events, endometrial cancer, and thromboembolic episodes. J Clin Oncol 16:2018-2024, 1998.
13. Thürlimann B, Keshaviah A, Coates AS, et al, for the Breast International Group (BIG) 1-98 Collaborative Group: A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747-2757, 2005.
14. Schroth W, Atoniadou L, Fritz P, et al: Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYPD6 and CYP2C19 genotypes. J Clin Oncol 25:5187-5193, 2007
15. Goetz MP, Knox SK, Suman VJ, et al: The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxfen. Breast Cancer Res Treat 101:113-121, 2007.
16. Gallicchio L, Tkaczuk K, Lord G, et al: Medication use, tamoxifen (TAM), and TAM metabolite concentrations in women with breast cancer. Cancer Lett 211:57-67, 2004.
17. Howell A, Cuzick J, Baum M, et al: Results of the ATAC (Arimidex, Tamoxifen, Alone of in Combination) trial after 5 years’ of adjuvant therapy for breast cancer. Lancet 365:60-62, 2005.
18. Eastell R, Hannon RA, Cuzick J, et al: Effect of an aromatase inhibitor on bone mineral density and bone turnover marker: 2-year results of the Anastrozole Tamoxifen Alone or in Combination (ATAC) trial. J Bone Miner Res 21:1215-1223, 2006.
19. Brufsky A, Harker WG, Beck JT, et al: Zolendronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol 25:829-836, 2007.
20. Early Breast Cancer Trialist’s Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet 351:1451-1467, 1998.
21. Owusu C, Lash TL, Silliman RA, et al: Effectiveness of adjuvant tamoxifen therapy among older women with early stage breast cancer. Breast J 13:374-382, 2007.
22. Coates AS, Keshaviah A, Thürlimann B, et al: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for post-menopausal women with endocrine-responsive early breast cancer: Update of Study BIG 1-98. J Clinical Oncology. 2007. 25,5; 486-92.
23. Coombes RC, Hall E, Gibson LJ, et al: A randomized trial of exemestane after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. N Engl J Med 350:1081-1092, 2004.
24. Goss PE, Ingle JN, Martino S, et al: A randomized trial of letrozole after 5 years of tamoxifen in postmenopausal women with early-stage breast cancer. N Engl J Med 349:1793-1802, 2003.
25. Goss PE, Ingle JN, Martino S, et al: Updated analysis of NCIC CTG MA.17 randomized placebo controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer (abstract 847). Proc Am Soc Clinc Oncol 23:87, 2004.
26. Elkin EB, Hurria A, Mitra N, et al: Adjuvant chemotherapy and survival in older women with hormone receptor negative breast cancer: Assessing outcome in a population-based observational cohort. J Clin Oncol 24:2757-2764, 2006.
27. Pritchard KI, Shepherd LE, O’Malley FP, et al: HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 354:2177-2179, 2006.
28. Berry DA, Cirrincione C, Henderson IC, et al: Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658-1667, 2006.
29. Paik S, Shak S, Tang G, et al: A multigene assay to predict recurrence of tamoxifen-treated node negative breast cancer. N Engl J Med 351:2817-2826, 2004.
30. Paik S, Tang G, Shak S, et al: Gene expression and benefit of chemotherapy in node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726-3734, 2006.
31. Albain K, Barlow W, Shak S, et al: Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal, node-positive, ER-positive breast cancer (abstract 10). Presented at the San Antonio Breast Symposium, 2007.
32. Henry NH, Hayes DF: Use of gene-expression profiling to recommend adjuvant chemotherapy for breast cancer. Oncology (Williston Park) 21:1301-1309, 2007.
33. Fargeot P, Bonneterre J, Roche H, et al: Disease free survival advantage of weekly epirubicin plus tamoxifen versus tamoxifen alone as adjuvant treatment of operable, node-positive, elderly breast cancer patients: 6-year follow-up results of the French Adjuvant Study Group 08 trial. J Clin Oncol 22:4674-4682, 2004.
34. Crivellari D, Bonnetti M, Castiglione-Gertsch M, et al: Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: The International Breast Cancer Study Group Trial VII. J Clin Oncol 18:1412-1422, 2000.
35. Viani GA, Afonso SL, Stefano EJ, et al: Adjuvant trastuzumab for treatment of her-2 positive early breast cancer: Meta-analysis of published randomized trials. BMC Cancer 7:153, 2007.
36. Smith I, Procter M, Gelber RD, et al: 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet 369:29-36, 2007.
37. Smith I, Procter M, Gelber R, et al: Trastuzumab after adjuvant chemotherapy in older patients (author’s reply). Lancet 369:991-992, 2007.
38. Balducci L: Cancer chemotherapy in elderly patients. J Oncol Manag 14:35-38, 2005.
39. Gomez H, Mas L, Casanova L, et al: Elderly patients with aggressive non-Hodgkin’s lymphoma treated with CHOP chemotherapy plus granulocyte-macrophage-colony stimulating factor: Identification of two age subgroups with differing hematologic toxicity. J Clin Oncol 16:2352-2358, 1998.
40. Zinzani PL, Pavone E, Storti S, et al: Randomized trial with or without granulocyte-colony stimulating factor as adjunct to induction VNCOP-B for elderly high grade non-Hodgkin’s lymphoma. Blood 89:3974-3979, 1997.
41. Hurria A, Brogan K, Panageas KS, et al: Patterns of toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat 92:151-156, 2005.
42. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology, v.1.2005 and v.1.2006. Available at www.nccn.org. Accessed Feb 5, 2008.
43. Lyman GH, Kuderer N, Agboola O, et al: Evidence based use of colony-stimulating factors in elderly cancer patients. Cancer Control 10:487-499, 2003.
44. Pinder MC, Duan Z, Goodwin JS, et al: Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 25:3808-3815, 2007.
45. Patt DA, Duan Z, Fang S, et al: Acute myeloid leukemia after adjuvant breast cancer therapy in older women: Understanding risk. J Clin Oncol 25:3871-3876, 2007.
46. Hughes KS, Schnaper LA, Berry D, et al: Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 351:971-977, 2004.
47. Extermann M, Meyer J, McGinnis M, et al: A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol 49:69-75, 2004.
48. Extermann M. Geriatric assessment with focus on instrument selectivity for outcomes. Cancer J 11:474-480, 2005.
49. Hurria A, Lachs MS, Cohen HJ, et al: Geriatric assessment for oncologists: Rationale and future directions. Crit Rev Oncol Hematol 59:211-217, 2006.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.