Anti-EGFR Mechanism of Action: Antitumor Effect and Underlying Cause of Adverse Events
Overexpression of the epidermal growth factor receptor (EGFR) is correlated with poor prognosis in many human cancers. Two main classes of anticancer agents affect the EGFR: those targeting the extracellular ligand-binding domain and those that block the intracellular tyrosine kinase (TK) domain. Cetuximab (Erbitux) is a mouse/human chimeric monoclonal antibody that targets the ligand-binding domain of the EGFR, whereas erlotinib (Tarceva) and gefitinib (Iressa) are small-molecule TK inhibitors. Common toxicities of agents targeting the EGFR differ from those associated with traditional chemotherapy. Given the common pathway through which these agents work, some adverse events are similar. Many patients treated with these agents develop an acne-like rash on the face and upper body, most likely related to keratinocyte alterations and hair follicle proliferation and maturation. Although clinical manifestation of this reaction closely resembles acne vulgaris, the histology is more similar to infectious folliculitis. Other adverse events appear to be related to a drug class or individual agent. For example, interstitial lung disease is a rare but potentially fatal reaction that has been reported with gefitinib. Hypomagnesemia reported in association with cetuximab may be related to EGFR blockade in the kidney. Anaphylactic or anaphylactoid infusion reactions are also seen with cetuximab, as with other monoclonal antibodies.
Overexpression of the epidermal growth factor receptor (EGFR) is correlated with poor prognosis in many human cancers. Two main classes of anticancer agents affect the EGFR: those targeting the extracellular ligand-binding domain and those that block the intracellular tyrosine kinase (TK) domain. Cetuximab (Erbitux) is a mouse/human chimeric monoclonal antibody that targets the ligand-binding domain of the EGFR, whereas erlotinib (Tarceva) and gefitinib (Iressa) are small-molecule TK inhibitors. Common toxicities of agents targeting the EGFR differ from those associated with traditional chemotherapy. Given the common pathway through which these agents work, some adverse events are similar. Many patients treated with these agents develop an acne-like rash on the face and upper body, most likely related to keratinocyte alterations and hair follicle proliferation and maturation. Although clinical manifestation of this reaction closely resembles acne vulgaris, the histology is more similar to infectious folliculitis. Other adverse events appear to be related to a drug class or individual agent. For example, interstitial lung disease is a rare but potentially fatal reaction that has been reported with gefitinib. Hypomagnesemia reported in association with cetuximab may be related to EGFR blockade in the kidney. Anaphylactic or anaphylactoid infusion reactions are also seen with cetuximab, as with other monoclonal antibodies.
The epidermal growth factor receptor (EGFR) is among the most widely studied growth factor receptors due to its ubiquity and pleiotropic signaling effects.[1,2] EGFR is expressed by nearly all adult human tissues, with the notable exception of hematopoietic cells.[2,3] Activation of EGFR results in a range of effects, including cell proliferation, differentiation, migration, adhesion, and inhibition of apoptosis.[4] It also enhances processes crucial to tumor growth and progression, such as angiogenesis, tumor invasiveness, and metastatic spread.[5] EGFR is overexpressed by many human cancers, while its signaling is upregulated due to autocrine stimulation in others.[1,5] Its expression has been shown to correlate with disease progression, reduced survival, poor response to treatment, and chemotherapy resistance in several human cancers.[5]
For these reasons, EGFR is a rational target for the design and development of novel agents that aim to distinguish malignant and nonmalignant cells. Two main categories of compounds have been developed: agents that target the extracellular domain of the receptor and those that target the intracellular tyrosine kinase (TK) domain (Table 1). Both approaches result in blockade of signal transduction, thereby preventing the downstream effects normally associated with EGFR activation.[4,5]
The monoclonal antibody cetuximab (Erbitux) is the first commercially available agent designed to target the EGFR extracellular domain; it is approved for use in patients with EGFR-expressing, metastatic colorectal cancer.[6] Gefitinib (Iressa) and erlotinib (Tarceva) are small-molecule inhibitors of the TK domain, each initially approved for use in patients with advanced non-small-cell lung cancer who have progressed on at least one (erlotinib) or two (gefitinib) prior chemotherapy regimens.[7,8] (As of June 2005, the US Food and Drug Administration has limited the commercial use of gefitinib to those patients currently receiving and benefiting from the drug or those who have received it and benefited from it in the past, pending the outcomes of additional studies.[9])
Despite the differences in chemical structure and mechanism of action of these two drug classes, many of the resultant side effects are similar due to their effects on a common target. This article further describes the EGFR and adverse event profiles of the agents that target it, with the goal of distinguishing adverse events related to EGFR inhibition from those unique to the individual drugs.
The Epidermal Growth Factor Receptor
Structure and Activation
The EGFR, also known as HER1 or ErbB1, is a 170-kD glycoprotein that consists of an extracellular ligand-binding domain, a hydrophobic transmembrane region, and an intracellular TK domain. Ligands for the EGFR include EGF, transforming growth factor-alpha (TGF-α), amphiregulin, heparin-binding EGF, betacellulin, and epiregulin.[4] EGFR exists as a monomer in the cell membrane. Upon ligand binding, individual receptors cluster on the cell surface, which facilitates dimerization.[10] EGFR can form a homodimer with another EGFR monomer or a heterodimer with another member of the erbB family (eg, HER2/neu, HER3, or HER4).[4,11] Dimerization is followed by TK activation and autophosphorylation on tyrosine residues, key steps to the initiation of downstream physiologic and pathogenic events (Figure 1). Ultimately signaling is “turned off” by internalization of receptor/ligand complexes.[4,5,12]
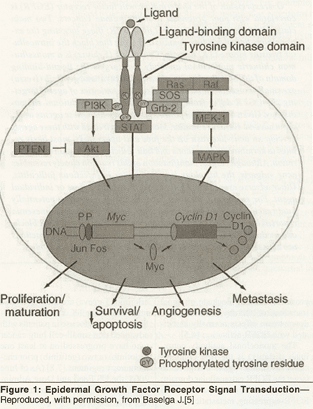
The precise signaling pathways that are activated in response to EGFR-ligand binding depend on both the activating ligand and coreceptor to which EGFR dimerizes.[4,13] Major pathways include the MAPK and PI-3K/Akt pathways.[5,11] The Ras-Raf-MEK-MAPK pathway produces cell proliferation and angiogenesis, inhibits apoptosis, and increases metastatic spread. The PI-3K/Akt pathway affects cell survival, metabolism, and proliferation, and it inhibits apoptosis.[11]
Physiologic Role of EGFR Signaling
Signaling by EGFR is critical for epithelial development, proliferation, and organogenesis.[4] Studies with EGFR knockout mice demonstrate that the lack of EGFR results in embryonic and perinatal mortality. If born, these mice survive for only days to weeks, and they exhibit widespread physical abnormalities, including severe impairment of epithelial development in multiple organ systems such as the gastrointestinal tract, lungs, brain, skin, eyes, kidneys, and liver.[4,11,14] There are defects in hair development, respiratory distress, retarded growth, and progressive wasting in these animals.[11] Conversely, activation of EGFR signaling via the administration of EGF enhanced the maturation of epithelial tissues in other murine studies, resulting in precocious eye opening and tooth eruption in newborn mice.[1]
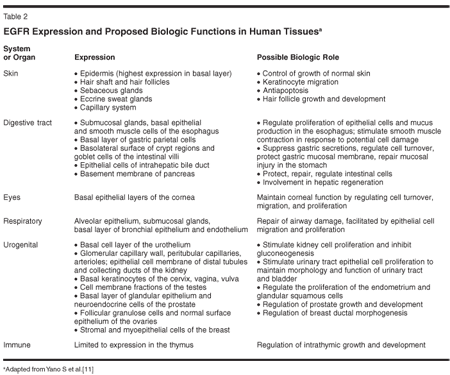
These findings suggest that EGFR signaling plays an important role in multiple organ systems. Expression of EGFR has been documented in tissues of the digestive tract, skin, eyes, thymus, and respiratory, urogenital, and endocrine systems (Table 2). No detectable levels of EGFR have been found in skeletal tissue, lymph nodes, spleen, or the central nervous or cardiovascular systems.[11] Despite the ubiquity of EGFR, its role appears to be most important in the developing organism. The mitotic effects of exogenous EGF on mouse keratinocytes diminish with increasing age of the animal. The expression of EGFR on human skin cells also diminishes with age. In human fetal epidermis EGFR expression persists in all cell layers, whereas in adults there is limited distribution in normal skin.[15] Indeed, in adults, EGFR expression is found primarily on epithelial cells with the highest proliferative potential-namely the basal keratinocytes-consistent with its role as a regulator of normal skin growth.[11,15,16]
Inhibition of EGFR
Anti-EGFR Agents: Mechanisms of Action
Interest in the EGFR as a target for cancer therapy began more than 20 years ago.[17] In the early 1980s, it was demonstrated that EGFR is the cellular homolog of the avian v-erbB oncogene.[4,17] Oncogenic transformation of the EGFR pathway occurs due to mutation, overexpression, and/or alteration of its normal regulatory mechanisms.[4] Protein overexpression and/or other EGFR alterations can occur in tumor cells; EGFR gene amplification has also been documented in some tumor specimens.[4,17] Solid tumors that have been demonstrated to overexpress EGFR include prostate, breast, gastric, colorectal, ovarian, non-small-cell lung, and head and neck cancers.[5,16,18]
Preclinical studies conducted with agents that block EGFR signaling validated the EGFR as a target for cancer drug development. These studies showed that EGFR-targeting agents could inhibit tumor growth and progression and reduce cellular proliferation, angiogenesis, and metastases.[5]
Cetuximab is a chimeric monoclonal antibody that recognizes the extracellular domain of the EGFR and thereby competes with its ligands for receptor occupation. Upon binding, cetuximab induces receptor dimerization and endocytosis, resulting in EGFR downregulation from the cell surface, cell-cycle arrest, and cell death.[4] Other studies have shown that cetuximab increases the expression of the cell-cycle inhibitor p27Kip1, induces apoptosis, and inhibits the production of angiogenic and metastatic factors in some cell lines.[5] Cetuximab produces synergy with chemotherapy and enhances the effects of radiation therapy.[5,16] As an immunoglobulin G1-derived monoclonal antibody, cetuximab may also produce cytotoxicity through immunologic mechanisms specific to that Ig subtype. By coating the EGFR with IgG1 molecules, cetuximab may induce or enhance innate immune effector mechanisms, such as antibody-dependent cellular cytotoxicity.[2,4] Whether this mechanism has relevance when cetuximab is administered to cancer patients who may be immunocompromised remains to be determined.[4]
The TK activity of EGFR is crucial for tumor progression, and the TK inhibitors (TKIs) that have been developed directly compete with ATP for the MgATP binding site of the EGFR TK catalytic domain.[4] Preclinical studies showed that gefitinib produced a number of relevant antitumor actions in vitro, including reduced cell proliferation, increased apoptosis, inhibition of cell migration and invasiveness, antiangiogenic activity, and induction of cell-cycle arrest. Gefitinib inhibited tumor growth in vivo in prostate, breast, ovarian, colorectal, and lung (small-cell and non-small-cell) models.[5] Tyrosine kinase inhibitors inhibit the growth of EGFR-expressing human cancer cell lines in vitro and produce additive or synergistic inhibition in combination with chemotherapy and radiation.[16]
Clinical Efficacy
Based on the scientific background, both anti-EGFR monoclonal antibodies and small-molecule TKIs entered the clinic for testing. In general, EGFR overexpression in tumors is associated with poor patient prognosis.[4] Early trials with drugs from each class showed promising activity against neoplasms that overexpress EGFR.[19-21] Pivotal clinical trials that led to the approval of cetuximab, erlotinib, and gefitinib are described elsewhere in this supplement. Additional research continues with these agents as well as other investigational antibodies and TKIs in numerous epidermoid cancers such as squamous cell carcinoma of the head and neck and glioblastoma.
Toxicities Associated With Inhibition of EGFR
Dermatologic toxicities are the most frequently reported side effects associated with the currently available anti-EGFR therapies. Infusion reactions are unique to monoclonal antibodies, which require intravenous administration, whereas diarrhea and interstitial lung disease (ILD) appear to be predominantly associated with TKIs. Hypomagnesemia, initially observed in patients with ovarian cancer, has recently been recognized as a relevant side effect of cetuximab therapy.[22,23] These events and their biologic basis are further described below.
Acneiform Rash/Acute Folliculitis
Skin toxicity-described as rash, acne, acneiform skin reaction, acneiform rash, or maculopapular skin rash-is common with all agents that target EGFR.[24] The frequency of rash reported in phase II non-small-cell lung cancer trials ranged from 43% to 54% with gefitinib, 67% with erlotinib, and 74% with cetuximab.[25] Skin reactions generally occur within the first 2 to 3 weeks of therapy and may resolve spontaneously during continued treatment.[24,26] The rash is characterized by a pustular/papular appearance. Pustules are generally sterile, although secondary infection can occur. Reactions are generally mild to moderate in severity. Severe reactions (ie, those interfering with activities of daily living) are less common.[24] The rash is most commonly seen on the upper body including the face, neck, scalp, chest, and upper back.[16,26] Rash eruptions may be accompanied by other cutaneous symptoms such as paronychia/nail disorders as well as trichomegaly or abnormal hair growth, particularly in the eyelashes.[27-29] Since these reactions are similar regardless of which agent is involved, dermatologic toxicity is probably a class effect.[30]
To better understand the biologic basis for these reactions, it is important to review the normal structure and function of human skin (Figure 2). The epidermis comprises several layers including the stratum corneum, the stratum granulosum, and the stratum basale (or basal layer). The stratum corneum is the outermost layer and provides the skin's barrier protection. It consists of anucleated, dead cells. The basal layer contains the proliferating keratinocytes that ultimately migrate through the other layers to form the stratum corneum upon terminal maturation. Contiguous with the epidermis are the outer root sheaths of the hair follicle, necessary for the formation of hair shafts.[31]
The EGFR is expressed predominantly on the basal keratinocytes in adult skin, where it regulates keratinocyte proliferation, differentiation, migration, and survival.[15,30] However, expression is also seen in hair follicles and preclinical studies demonstrate a prominent role for the EGFR in follicular homeostasis.[25,32] Studies with EGFR knockout mice confirm that EGFR signaling has a significant role in the normal development of hair follicles and skin.[14,33] These mice show a disrupted terminal differentiation of the epidermis and hair follicles.[11] Mice with null or mutant EGFR develop skin abnormalities, follicular necrosis, and thinning hair followed by alopecia.[25,32,34] Inflammatory mediators are found in the skin.[25] The inflammatory response may be due to a strong immune response to follicular degeneration and destruction; the remains of the hair follicle may be recognized as a foreign substance.[34]
The histologic response in humans is consistent with preclinical findings. The most common histologic finding in the skin from patients who develop a rash while receiving an anti-EGFR TKI or monoclonal antibody is neutrophilic infiltration.[25] Neutrophils are especially prominent around the hair follicles and sweat glands.[16] There is no change from baseline in skin thickness during treatment, but the stratum corneum is thinner and no longer exhibits its normal basketweave pattern.[31] The hair follicles are enlarged and blocked by excess keratin-ocytes.[25,30,31] In most cases, no infectious organisms are found, although microorganisms have been found in follicular plugs.[24,30]
Although the reaction resembles acne vulgaris clinically, particularly given its common appearance on the face and upper body, the microcomedones and comedones that characterize acne vulgaris are not seen. Rather, the intrafollicular collection of neutrophils more closely resembles infectious folliculitis, although the precise etiology remains to be determined. Indeed, an expert panel of oncologists and dermatologists familiar with anti-EGFR therapy recently suggested that this reaction may represent an entirely new dermatologic entity.[24]
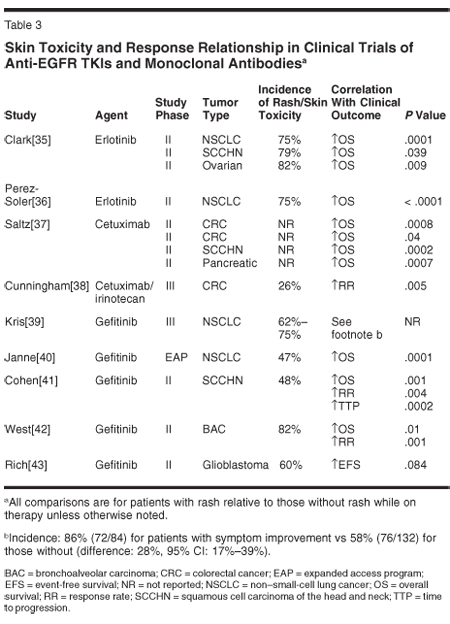
A correlation between the occurrence of skin toxicity and response and clinical outcomes has been reported by several investigators (Table 3). These data are compelling, but more research is needed to draw firm conclusions. While it seems likely that skin reactions are a surrogate for antitumor activity, another possibility is that these reactions may be surrogates for immunocompetence since the reaction appears to involve an immune-mediated inflammatory response.[36] Moreover, since cutaneous toxicity has been relatively uncommon with traditional cytotoxic agents, it is possible that in early trials with anti-EGFR TKIs and monoclonal antibodies, clinicians were inconsistent when classifying and grading these events. An improved categorization system and evaluation of the relationship between the grade of toxicity and outcome are needed.
In addition, the potential for a relationship between rash and response challenges the current dosing paradigm, suggesting that dosing anti-EGFR therapy until rash development may be the optimal strategy. Clinical trials designed with such a dose-escalation scheme are underway with erlotinib in non-small-cell lung cancer and glioblastoma.[24] A similar hypothesis is being investigated in the EVEREST study, in which colorectal cancer patients with no rash or grade 1 rash will be randomized to continue with cetuximab/irinotecan therapy at currently recommended doses or to receive an escalated dose of cetuximab in combination with irinotecan.[44]
Interstitial Lung Disease
Interstitial lung disease has emerged as a rare but serious adverse event associated with EGFR inhibitors. Although reported with all agents, the incidence appears highest with gefitinib therapy. Specifically, the incidence has been estimated as 1.9% with gefitinib based on data from more than 19,000 patients treated in Japan and as 3.5% based on retrospective data from 1,976 Japanese patients collected as part of a specific epidemiologic study of gefitinib-induced ILD.[45] The mortality rate has been reported as 0.6% and 1.6%, respectively. The incidence of ILD appears to be lower in the United States (0.3%, based on 23,000 patients treated in an expanded access program).[46] Interstitial lung disease has been reported in association with erlotinib therapy, although the incidence rate has not differed between patients treated with erlotinib or placebo in clinical trials (0.8% in both groups).[8] Similarly, rare reports of ILD have occurred with cetuximab (3/774 or < 0.5%), and treatment should be interrupted in the event of acute onset or worsening pulmonary symptoms until ILD can be confirmed or ruled out.[6]
Clinically, ILD has been defined as a syndrome consisting of dry cough, fever, and hypoxia accompanied by interstitial pulmonary infiltrates on chest x-ray or CT. Based on radiologic findings, ILD can generally be classified as acute interstitial pneumonia, bronchiolitis obliterans organizing pneumonia, or acute eosinophilic pneumonia.[47] Retrospective studies designed to identify risk factors for ILD suggest that preexisting pulmonary fibrosis, preexisting hypoxia, poor performance status, male gender, hypoalbuminemia, and history as a current or former smoker are significant risk factors.[45,47,48] The prognosis is particularly poor for patients with poor performance status and for those developing ILD within 2 weeks of gefitinib initiation.[45]
The precise mechanism behind treatment-associated ILD has not been elucidated. It has been postulated that EGFR inhibition may have a damaging effect on lung tissue related to decreased expression of the pulmonary surfactant-associated protein (SP-A). Epidermal growth factor stimulates SP-A synthesis in fetal lung tissue, and preclinical studies have shown that EGFR inhibition with TKIs or antisense agents decreases SP-A expression in vitro and in vivo.
Tamura and colleagues[49] compared baseline SP-A protein expression levels in cancer tissue and normal bronchial epithelial cells taken from patients who later developed ILD during gefitinib therapy with those who did not develop ILD. None of the original tumor specimens from the 10 patients with ILD expressed SP-A, while 5 of 10 specimens from patients without ILD did express the protein (P < .05). SP-A expression in normal bronchial epithelial cells was seen in 2 of 10 patients with ILD and in 8 of 10 patients without ILD (P < .05). They concluded that low baseline levels of SP-A expression in tumor cells and normal bronchial cells are associated with development of ILD during gefitinib therapy. Further research is needed to identify other predictive markers, which would ideally be useful in guiding treatment decisions.
Infusion Reactions
Infusion reactions are common side effects associated with monoclonal antibody therapy. Cetuximab is a recombinant human/mouse chimeric antibody. It is composed of the Fv regions of a murine antibody to EGFR and human IgG1 heavy and kappa light chain constant regions. The incidence of severe infusion reactions is estimated to be 3%, although these reactions are rarely fatal (< 0.01%).[6] Ninety percent of reactions occur with the first administration of cetuximab. Severe reactions are characterized by the rapid onset of airway obstruction, urticaria, and hypotension and are managed by prompt discontinuation of therapy. Treatment with epinephrine, corticosteroids, intravenous antihistamines, bronchodilators, and oxygen should be considered based on the severity of the reaction.
Infusion reactions are hypersensitivity manifestations that are immunologic in nature. The pathogenesis of infusion reactions in patients is probably linked to the development of anti-idiotypic antibodies triggered by the murine portion (Fv) of recombinant monoclonal antibody. Murine, chimeric, and humanized monoclonal antibodies all contain murine protein to some extent.[50] Indeed, the clinical development of monoclonal antibodies was initially limited due to the immunogenicity associated with pure murine proteins and resultant production of human anti-mouse antibodies (HAMA).[51] Chimeric monoclonal antibodies were developed to reduce the immunogenicity associated with monoclonal antibody therapy; however, some patients develop human anti-chimeric antibodies (HACA). Similarly, even with humanized or completely human monoclonal antibodies, some patients still develop human anti-human antibodies (HAHA) that, in theory, could induce infusion reactions or limit efficacy.[50]
The precise immune mediators responsible for the infusion reactions to cetuximab have not yet been identified. Reactions to cetuximab have been described as anaphylactic and anaphylactoid in nature in clinical trials.[19,26] Some authors have indicated that the term “infusion reaction” may be grouping together different immune entities, including true allergic reactions (although these are believed to be rare).[52] In any case, there are no published data supporting or refuting a role for IgE in the development of infusion reactions to cetuximab. Moreover, 4 of 120 treated patients developed detectable HACA levels after treatment, although none of these patients experienced an allergic or anaphylactic reaction to treatment.[53]
Cetuximab does not target circulating cells, which suggests that the reticuloendothelial system (responsible for removal of the circulating B cell/monoclonal antibody complex in the case of the anti-CD20 monoclonal antibody rituximab [Rituxan], and probably linked to the reactions caused by the release of cytokines from the cells themselves and from tissue macrophages in rituximab patients[52]) is not involved. Since the majority of cases occur during the first infusion, it is possible that previous sensitization to murine immunoglobulin may be a contributing factor; exposure and sensitivity to mouse allergens is relatively common among children living in urban cities, although exposure among cancer patients is not known.[54] Additional studies are needed to evaluate the potential roles of IgE, IgG, and vasoactive substances such as complement and histamine in the development of infusion reactions during cetuximab treatment.
Efforts to further reduce the immunogenicity of monoclonal antibodies that target EGFR include the development of humanized or fully human monoclonal antibodies. Panitumumab (ABX-EGF), a fully human antibody targeting EGFR, and matuzumab (EMD72000), a humanized antibody against EGFR, are in clinical development. Infusion reactions to panitumumab have been extremely rare, reported in no patients in two early trials and in just one patient with colorectal cancer and one patient with non-small-cell lung cancer in other studies.[55-58] No patients in the latter two studies developed HAHA.[57,58] Allergic or infusion reactions to matuzumab have not been reported in trials to date.[59]
Hypomagnesemia
Hypomagnesemia has recently emerged as a relatively common side effect of cetuximab therapy. Although not specifically identified as an adverse event during clinical trials, magnesium depletion has been reported to occur in up to half of patients treated with cetuximab, alone or in combination with chemotherapy, in current clinical trials (N = 224).[23] A retrospective review of 154 consecutive patients treated for colorectal cancer at a single institution found 34 patients for whom serum magnesium had been determined during treatment; 24% of these patients developed grade 3/4 hypomagnesemia (n = 6 for grade 3; n = 2 for grade 4).[22]
Schrag and colleagues[22] postulate that cetuximab-induced magnesium depletion may be related to the high expression of EGFR in the kidney. After filtration through the glomerulus, free extracellular magnesium is reabsorbed in the thick ascending limb of the loop of Henle (approximately 70%). The remainder is reabsorbed in the proximal and distal tubules. EGFR is highly expressed in the apical membrane of the loop of Henle and the distal tubules. Schrag et al suggest that cetuximab-induced EGFR blockade in the nephron reversibly impairs the active transport of magnesium via interference with a specific cation channel, TRPM6. However, effects on the gut absorption of magnesium cannot be ruled out at the present time. Whether TKIs produce clinically significant hypomagnesemia remains to be determined.[7,8]
Conclusions
The TKIs erlotinib and gefitinib and the monoclonal antibody cetuximab share a common target, the EGFR. Although the precise mechanism of action may differ between these two classes of drugs, each ultimately inhibits EGFR signaling and the related downstream biologic effects. The development of skin reactions that resemble acne or folliculitis appears to be a class effect related to EGFR inhibition. Preclinical and clinical data support a role for the disruption of normal epidermal and follicular development in the pathogenesis of these reactions. Indeed, the development of rash may prove to be a strong surrogate marker for efficacy, as it is associated with improved response rates and overall survival in several clinical trials.
Other clinically important adverse events may be related to the specific agent rather than to the therapeutic class. An increased incidence of ILD has been reported predominantly with gefitinib, although it can occur during treatment with any of the agents. While the etiology of ILD remains to be elucidated, potential risk factors have been identified in retrospective studies. Hypomagnesemia has been reported with cetuximab, and patients should be monitored for electrolyte disturbances during and after cetuximab therapy. Reactions related to cetuximab infusion are similar to those seen with the administration of other monoclonal antibodies with a murine component. The success of investigational humanized or fully human monoclonal antibodies that are currently in clinical trials may obviate concern about this adverse event in the future.
Disclosures:
Dr. Lenz has done consulting for and served on advisory boards and speakers bureaus for Bristol-Myers Squibb and Genentech. He has also served on advisory boards for ImClone.
References:
1. Wells A: Molecules in focus: EGF receptor. Int J Biochem Cell Biol 31:637-643, 1999.
2. Bier H, Hoffman T, Haas I, et al: Anti-(epidermal growth factor) receptor monoclonal antibodies for the induction of antibody-dependent cell-mediated cytotoxicity against squamous cell carcinoma lines of the head and neck. Cancer Immunol Immunother 46:167-173, 1998.
3. Miettinen PJ, Berger JE, Meneses J, et al: Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376:337-341, 1995.
4. Arteaga CL: The epidermal growth factor receptor: From mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol 19:32s-40s, 2001.
5. Baselga J: Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist 7(suppl 4):2-8, 2002.
6. Erbitux (Cetuximab) package insert. ImClone Systems, NY, and Bristol-Myers Squibb, Princeton, NJ; August 2005.
7. Iressa (gefitinib) tablets [package insert]. AstraZeneca Pharmaceuticals, Wilmington, DE; November 2004.
8. Tarceva (erlotinib) tablets [package insert]. Genentech, South San Francisco, CA, and OSI Pharmaceuticals, Melville, NY; 2004.
9. Food and Drug Administration: FDA public health advisory: New labeling and distribution program for gefitinib (Iressa). Available at http://www.fda.gov/cder/drug/infopage/gefitinib/default.htm. Accessed February 28, 2006.
10. Salomon DS, Brandt R, Ciardiello F, et al: Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19:183-232, 1995.
11. Yano S, Kondo K, Yamaguchi M, et al: Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res 23:3639-3650, 2003.
12. Herbst RS, Shin DM: Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: A new paradigm for cancer therapy. Cancer 94:1593-1611, 2002.
13. Moghal N, Sternberg PW: Multiple positive and negative regulators of signaling by the EGF-receptor. Curr Opin Cell Biol 11:190-196, 1999.
14. Threadgill DW, Dlugosz AA, Hansen LA, et al: Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science 269:230-234, 1995.
15. Nanney LB, Stoscheck CM, King LE, et al: Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol 94:742-748, 1990.
16. Bunn PA, Franklin W: Epidermal growth factor receptor expression, signal pathway, and inhibitors in non-small cell lung cancer. Semin Oncol 29(suppl 14):38-44, 2002.
17. Mendelsohn J: Blockade of receptors for growth factors: An anticancer therapy. The Fourth Annual Joseph H. Burchenal American Association for Cancer Research clinical research award lecture. Clin Cancer Res 6:747-753, 2000.
18. Grandis JR, Melhem MF, Barnes EL, et al: Quantitative immunohistochemical analysis of transforming growth factor-a and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 78:1284-1292, 1996.
19. Saltz LB, Meropol NJ, Loehrer PJ, et al: Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201-1208, 2004.
20. Hidalgo M, Siu LL, Nemunaitis J, et al: Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19:3267-3279, 2001.
21. Herbst RS, Maddox AM, Rothenberg M, et al: Selective oral epidermal growth factor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: Results of a phase I trial. J Clin Oncol 20:3815-3825, 2002.
22. Schrag D, Chung KY, Flombaum C, et al: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst 2005 97:1221-1224.
23. Food and Drug Administration. 2005 Safety Alerts for Drugs, Biologics, Medical Devices, and Dietary Supplements. Available at http://www.fda.gov/medwatch/safety/2005/safety05.htm#Erbitux. Last accessed February 9, 2006.
24. Perez-Soler R, Delord JP, Halpern A, et al: HER1/EGFR inhibitor-associated rash: Future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist 10:345-356, 2005.
25. Perez-Soler R: Can rash associated with HER1/EGFR inhibition be used as a marker of treatment outcome? Oncology 17(suppl):23-28, 2003.
26. Needle MN: Safety experience with IMC-C225, an anti-epidermal growth factor receptor antibody. Semin Oncol 29(suppl 14):55-60, 2002.
27. Bouche O, Brixi-Benmansour H, Bertin A, et al: Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol 16:1711-1172, 2005.
28. Boucher KW, Davidson K, Mirakhur B, et al: Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J Am Acad Dermatol 45:632-633, 2002.
29. Dueland S, Sauer T, Lund-Johansen F, et al: Epidermal growth factor receptor inhibition induces trichomegaly. Acta Oncol 42:345-346, 2003.
30. Herbst RS, LoRusso PM, Purdom M, et al: Dermatologic side effects associated with gefitinib therapy: Clinical experience and management. Clin Lung Cancer 4:366-369, 2003.
31. Albanell J, Rojo F, Averbuch S, et al: Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: Histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 20:110-124, 2002.
32. Kimyai-Asadi A, Jih MH: Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors [letter]. Arch Dermatol 138:129-130, 2002.
33. Sibilia M, Wagner EF: Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269:234-238, 1995.
34. Murillas R, Larcher F, Conti CJ, et al: Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J 14:5216-5223, 1995.
35. Clark GM, Perez-Soler R, Siu L, et al: Rash severity is predictive of increased survival with erlotinib HCl (abstract 786). Proc Am Soc Clin Oncol 22:196, 2003.
36. Perez-Soler R, Chachoua A, Hammond LA, et al: Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 16:3238-3247, 2004.
37. Saltz L, Kies M, Abbruzzese JL, et al: The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies (abstract 817). Proc Am Soc Clin Oncol 22:204, 2003.
38. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
39. Kris MG, Natale RB, Herbst RS, et al: Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small-cell lung cancer: A randomized trial. JAMA 290:2149-2158, 2003.
40. Janne PA, Gurubhagavatula S, Yeap BY, et al: Outcomes of patients with advanced non-small-cell lung cancer treated with gefitinib (ZD1839, Iressa) on an expanded access study. Lung Cancer 44:221-230, 2004.
41. Cohen EEW, Rosen F, Stadler WM, et al: Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 21:1980-1987, 2003.
42. West HL, Franklin WA, Gumerlock PH, et al: Gefitinib (ZD 1839) therapy for advanced bronchioloalveolar lung cancer (BAC): Southwest Oncology Group (SWOG) Study S0126 (abstract 7014) [and slide presentation]. Proc Am Soc Clin Oncol 22:14S, 2004.
43. Rich JN, Reardon DA, Peery T, et al: Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 22:133-142, 2004.
44. Segaert S, Van Cutsem E: Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16:1425-1433, 2005.
45. Seto T, Yamamoto N: Interstitial lung diseases (ILD) induced by gefitinib in patients with advanced non-small cell lung cancer (NSCLC): Results of a West Japan Thoracic Oncology Group (WJTOG) epidemiological survey (abstract 7064) [and slide presentation]. Proc Am Soc Clin Oncol 23:629, 2004.
46. Shah NT, Kris MG, Pao W, et al: Practical management of patients with non-small-cell lung cancer treated with gefitinib. J Clin Oncol 23:165-174, 2005.
47. Hotta K, Harita S, Bessho A, et al: Interstitial lung disease (ILD) during gefitinib treatment in Japanese patients with non-small cell lung cancer (NSCLC): Okayama Lung Cancer Study Group (abstract 7063). Proc Am Soc Clin Oncol 23:629, 2004.
48. Nakagawa M, Teramukai S, Tada H, et al: Hypoalbuminemia as a risk factor of interstitial lung disease (ILD) during gefitinib treatment in patients with non-small cell lung cancer (NSCLC): A JMTO study (abstract 7190). J Clin Oncol 23(suppl 16S):667s, 2005.
49. Tamura K, Okamoto I, Kurata T, et al: Low expressions of surfactant-associated protein (SP-A) in cancer tissues or in normal bronchial epithelial cells by immuno-histochemistry predict interstitial lung diseases (ILDs) induced by gefitinib in patients with advanced non-small cell lung cancer (NSCLC) (abstract 7188) [and slide presentation]. J Clin Oncol 23(suppl 16S):667s, 2005.
50. Cheifetz A, Mayer L: Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med 72:250-256, 2005.
51. Cheng JD, Adams GP, Robinson MK, et al: Monoclonal antibodies, in DeVita VT, Hellman S, Rosenberg SA (eds): Cancer: Principles and Practice of Oncology, pp 445-456, 7th ed. Philadelphia, Lippincott Williams & Wilkins, 2005.
52. Dillman RO: Infusion reactions associated with the therapeutic use of monoclonal antibodies in the treatment of malignancy. Cancer Metastasis Rev 18:465-471, 1999.
53. Khazaeli MB, LoBuglio AF, Falcey JW, et al: Low immunogenicity of a chimeric monoclonal antibody (MoAb), IMC-C225, used to treat epidermal growth factor receptor-positive tumors (abstract 808). Proc Am Soc Clin Oncol 19:207a, 2000.
54. Phipatanakul W, Eggleston PA, Wright EC, et al: Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol 106:1075-1080, 2000.
55. Rowinsky EK, Schwartz GH, Gollob JA, et al: Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol 22:3003-3015, 2004.
56. Weiner LM, Belldegrun A, Rowinsky E, et al: Updated results from a dose and schedule study of panitumumab (ABX-EGF) monotherapy, in patients with advanced solid malignancies (abstract 3059). J Clin Oncol 23(suppl 16S):206s, 2005.
57. Hecht JR, Patnaik A, Malik I, et al: ABX-EGF monotherapy in patients (pts) with metastatic colorectal cancer (mCRC): An updated analysis (abstract 3511). Proc Am Soc Clin Oncol 23:248, 2004.
58. Crawford J, Sandler AB, Hammond LA, et al: ABX-EGF in combination with paclitaxel and carboplatin for advanced non-small cell lung cancer (NSCLC) (abstract 7083). Proc Am Soc Clin Oncol 23:634, 2004.
59. Vanhoefer U, Tewes M, Rojo F, et al: Phase I study of the humanized anti-
epidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumors that express the epidermal growth factor receptor. J Clin Oncol 22:175-184, 2004.