Antifungal Resistance: The Clinical Front
The emergence of resistance and changes in the spectrum ofCandida infections have led to an increased interest in susceptibilitytesting of antifungal drugs. Such testing may be particularly useful inpatients with invasive candidiasis who have been previously treated withazole antifungals, those whose infections are not responding to treatment,and those with infections caused by non-albicans species of Candida.The choice of a specific antifungal depends on the clinical statusof the patient, the relative toxicity and efficacy of the drug in the givenpatient population, the infecting species and antifungal susceptibilityof the isolate, and the patient’s prior exposure to antifungal agents.Infectious Diseases Society of America recommendations for the initialmanagement of candidemia and acute disseminated candidiasisinclude an azole, caspofungin, amphotericin B (AmB), or a combinationof fluconazole plus AmB. Caspofungin and voriconazole show goodactivity against most Candida species and may be good alternatives forpatients with Candida glabrata and Candida krusei infections and forthose with relapsing infections.
The emergence of resistance and changes in the spectrum of Candida infections have led to an increased interest in susceptibility testing of antifungal drugs. Such testing may be particularly useful in patients with invasive candidiasis who have been previously treated with azole antifungals, those whose infections are not responding to treatment, and those with infections caused by non-albicans species of Candida. The choice of a specific antifungal depends on the clinical status of the patient, the relative toxicity and efficacy of the drug in the given patient population, the infecting species and antifungal susceptibility of the isolate, and the patient's prior exposure to antifungal agents. Infectious Diseases Society of America recommendations for the initial management of candidemia and acute disseminated candidiasis include an azole, caspofungin, amphotericin B (AmB), or a combination of fluconazole plus AmB. Caspofungin and voriconazole show good activity against most Candida species and may be good alternatives for patients with Candida glabrata and Candida krusei infections and for those with relapsing infections.
The recent shift in the spectrum of Candida infections, including the increased prevalence of non-albicans species and the emergence of intrinsically resistant strains, is pressing the clinician to reevaluate diagnostic and therapeutic strategies for invasive fungal infections. There is a clear and unequivocal trend in the emergence of both primary and secondary antifungal resistance,[1-3] although the full scope of the clinical implications of this trend remains unknown. Important lessons have been learned from antimicrobial resistance and, while parallels exist, there are unique concerns in the setting of fungal infections, where the topic of resistance is not fully appreciated. For example, the incidence of Candida glabrata candidemia has significantly increased among patients in the ICU.[2] Has this resulted in increased disease burden? In cancer patients, yes: candidemia caused by C glabrata has been associated with increased mortality.[4] Whether this trend is generalizable to other patient groups has not yet been established. In addition, there are differences among the Candida species, and somestrains are more virulent than others. For example, Candida dubliniensis is less pathogenic than C albicans, and reduced susceptibility of this strain to azole drugs can be readily induced in vitro and has been observed in many clinical isolates.[5] Is the increased prevalence of C dubliniensis cause for concern in light of its lower virulence? Candida krusei has been considered to be relatively avirulent, on the basis of animal data; however, it is associated with a mortality rate as high as 60% in patients with candidemia.[ 6-8] Such observations raise the question of whether there is a need for routine susceptibility testing and, if there is, what the clinical relevance of the available tests is. While these and other questions remain open to debate and are being investigated, certain practice guidelines and clinical observations are available. These guidelines and observations, which are based on surveillance and clinical data, provide a rational risk stratification for therapeutic decisions for the management of invasive candidiasis and will be the focus of this review.
Susceptibility TestingGuidelines for Testing
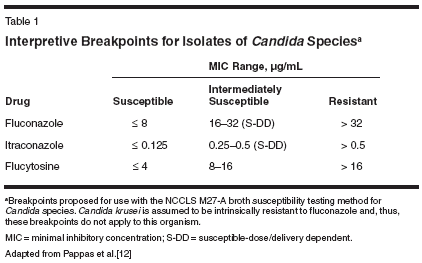
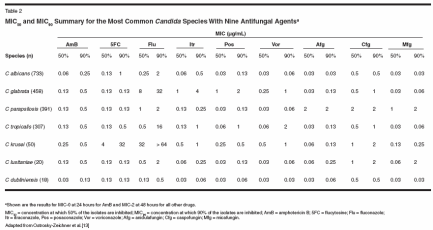
There has been a heightened interest in designing accurate methodologies for in vitro susceptibility testing of antifungal drugs. This interest is primarily fueled by the emergence of uncommon species, the increasing number of reports documenting drug resistance, and the introduction of new agents for which breakpoints have not been established. In response to these trends, the National Committee for Clinical Laboratory Standards (NCCLS) defined a standard reference broth microdilution method known as M27-A that provides validated breakpoints for interpretive classification of in vitro susceptibility of yeasts to antifungal drugs.[9,10] The subsequent document published in 2002, M27-A2, aims to achieve reproducible test results among institutions and specifies standardization of media, inocula, laboratory conditions, and drug solutions.[11] Interpretive breakpoints using the NCCLS M27-A method are available for testing the susceptibility of Candida species to three antifungal compounds: fluconazole, itraconazole, and flucytosine (Table 1).[12] An appreciation of how such data are generated and interpreted may be important to the clinician in that it ensures appropriate clinical application. Susceptibility is reported either qualitatively-as susceptible, intermediate,or resistant-or quantitatively, as the minimal inhibitory concentration (MIC) for an isolate when grown in the presence of an antifungal agent. The category "susceptibility-dose/ delivery dependent" was introduced to identify isolates with intermediate susceptibilities, and it underscores the importance of maximizing dosages and bioavailability to achieve successful outcomes.[12] For fluconazole, for example, the typical daily dose is 400 mg or higher, and blood levels as high as 32 μg/mL and above are required for successful therapy. Oral absorption of itraconazole is somewhat unpredictable, so it may be necessary to achieve blood levels of at least 0.5 μg/mL.[12] Flucytosine is given at dosages of 50 to 150 mg/kg/d, and blood levels of 16 μg/mL and higher may be desirable. In patients with normal renal function, these levels are easily achieved with a daily flucytosine dose of 100 mg/kg. Unfortunately, interpretive breakpoints have not been established for many antifungals, including amphotericin B (AmB) and its newer lipidbased formulations, expandedspectrum triazoles (voriconazole, posaconazole, and ravuconazole), and echinocandins (caspofungin, micafungin, and anidulafungin). However, recent MIC data for most of these compounds are available for the major Candida species (Table 2).[13] Susceptibility characteristics for these species are presented in Table 3.[12] Other methodologies for in vitrosusceptibility testing have been developed. The European Committee on Antibiotic Susceptibility Testing (EUCAST) microdilution is based on the M27-A reference procedure, but the incubation period is shortened from 48 to 24 hours.[14] The susceptibility test results obtained by the NCCLS and EUCAST procedures have been compared. Both procedures were used to test a panel of 109 bloodstream isolates of 5 Candida species against AmB, flucytosine, fluconazole, and itraconazole; an overall 92% agreement was reported for MICs obtained after the respective recommended incubation periods.[14] Several products using variations of these microdilutions are commercially available and may be preferred by the clinician, since these products are claimed to be easier to use and yield more rapid results. However, caution is necessary, since some of these products have not been shown to be reliable. Fluconazole susceptibility was evaluated in 800 clinical Candida isolates (60% C albicans) and two control strains (C krusei ATCC 6258 and Candidaparapsilosis ATCC 22019), using six commercial products and the NCCLS M27-A method (the gold standard).[ 9] The overall rates of agreement between the reference method and the commercial products ranged from 82% for Etest to 22% for Candifast. Four of the six commercial products showed a high percentage of concordance with the reference method for C albicans and C parapsilosis isolates, whereas all six had very low agreement rates for C glabrata, C krusei, and Candida tropicalis isolates.[9] Potential Role in Patient Care
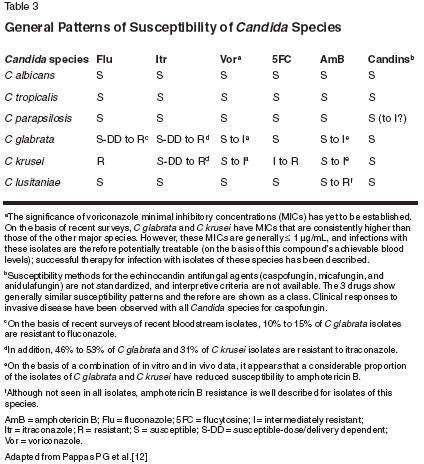
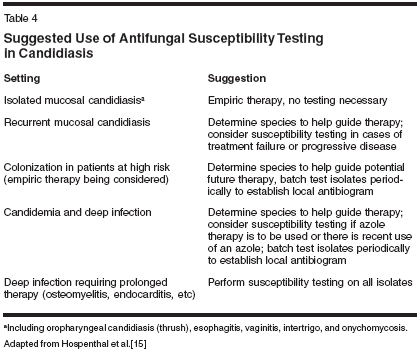
While not a standard of care in many clinical settings, in vitro susceptibility testing has predictive value for clinical response and may be particularly useful in patients previously treated with azole antifungals, those not responding to treatment, and those with infections caused by non albicans species of Candida. The main limitation of testing is the unpredictability of results with variations in assay conditions; nonetheless, identification of the infecting species pre-dicts likely susceptibility and may be a useful guide to therapy (Table 4).[11,12,15]
The importance of three factors- the local epidemiology of the species, the characteristics of the host, and the characteristics of the isolate-cannot be overemphasized in the correlation of MICs with treatment outcomes. These factors should be considered in any antifungal treatment paradigm.
- Local Epidemiology-Surveillance studies have shown important regional and institutional differences in species prevalence and susceptibility patterns, which are most likely related to specific selection pressures and infection control practices.[ 1,2,16,17] An international surveillance study of 306 episodes of candidemia in 34 medical centers (22 in the United States, 6 in Canada, and 6 in South America) revealed that the distribution of species varied markedly by country.[18] In the United States, 43.8% of bloodstream infections were caused by non-albicans species, with C glabrata being the most common. In Canada, the proportion of non-albicans bloodstream infections was slightly higher (47.5%), and C parapsilosis was the most common non-albicans species.[18]
Similarly, treatment choices may be different in a cancer institute, where most patients have received fluconazole prophylaxis and the prevalence of azole-resistant C albicans is increased, compared with a medical ward or ICU, where C parapsilosis associated with vascular catheters may be more prevalent.
- Host Factors-Knowledge of specific host risk factors, such as underlying disease, the presence of intravascular catheters, concurrent medications, and drug interactions, is paramount to achieving successful outcomes. An analysis of 37 studies of the distribution of Candida published between 1952 and 1992 found that C albicans or C tropicalis was more often reported in patients with leukemia than in those with other types of cancer.[19] Bone marrow transplant recipients were more likely to be infected by C krusei or Candida lusitaniae.[19]
- Characteristics of the Isolate- Therapeutic failures often stem from incorrect diagnoses. Thus, identification of the causative species can be of great value in guiding therapeutic choices. Unfortunately, in many cases of invasive candidiasis, the clinician does not have the luxury of time to await laboratory results before initiating therapy. A broad-spectrum azole or, in patients exposed to azoles, an alternative class of agents may represent the proper strategy for many patients. When antifungal resistance is unknown and/or the fungi in question are unusual, susceptibility testing may be particularly useful. If the organism is growing in the presence of large amounts of a particular drug, it is highly unlikely that that drug will be effective in the clinical setting.
Treatment DecisionsIDSA Guidelines for the Treatment of Candidiasis
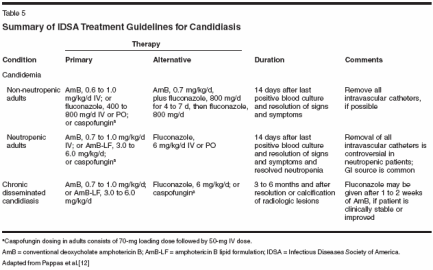
Updated treatment guidelines for candidiasis that are based on the strength of the supporting evidence and the quality of the underlying data have been issued by the Infectious Diseases Society of America (IDSA) (Table 5).[12] While these guidelines provide an evidence-based approach, the choice of a specific antifungal agent depends on a number of variables, including:
- The clinical status of the patient and the presence of organ dysfunction, which might affect drug clearance
- The relative toxicity and efficacy of the available antifungal drugs in the given patient population
- Knowledge of the infecting species and antifungal susceptibility of the isolate
- The patient's prior exposure to antifungal agents
Viable options for the initial management of candidemia and acute disseminated candidiasis include an azole, caspofungin, AmB, or a combination of fluconazole plus AmB.[12] However, C glabrata has reduced susceptibility to older azoles and AmB, and C krusei is resistant to fluconazole and has decreased susceptibility to AmB; therefore, caspofungin or voriconazole may be good alternatives in cases of identified C glabrata and C krusei infections. An analysis of the susceptibilities of 2,000 bloodstream Candida isolates to several antifungal agents revealed voriconazole and caspofungin to have potent antifungal activity against all Candida species, including C glabrata and C krusei.[13] However, I have recently observed multiple cases of C glabrata fungemia in patients receiving voriconazole. Thus, in patients recently exposed to azole therapy, it may be appropriate to start with an agent from a different class. In neutropenic patients with persistent fever and fungemia, AmB has traditionally been the therapy of choice[19]; however, among the newer agents, voriconazole[20] and caspofungin[ 21,22] have shown benefit in treatment. Antifungal Prophylaxis
Prophylaxis with azole antifungals, particularly fluconazole, has played an impressive role in reducing the number of systemic fungal infections, especially those caused by C albicans in patients at high risk, such as those with neutropenic cancer or those undergoing bone marrow transplantation.[ 23-25] A meta-analysis of 38 trials including 7,014 patients showed that antifungal prophylaxis with azoles (fluconazole, itraconazole, ketoconazole, and miconazole) or intravenous AmB reduced morbidity and mortality related to fungal infection in patientswith malignant disease and prolonged neutropenia and in recipients of hematopoietic stem cell transplants.[ 26]
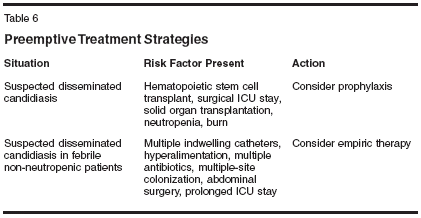
Both fluconazole and itraconazole can decrease Candida colonization and infection and fungal infection- related mortality; the lack of a consistent effect on overall mortality is most likely attributable to the severity of the underlying disease.[27-29] While fluconazole has the advantage of low toxicity, it is not active against some non-albicans species of Candida, and the likelihood of breakthrough infections with less-susceptible species remains. Further, C glabrata has been shown to become resistant to fluconazole with prolonged exposure in hematopoietic stem cell transplant recipients, leading to persistent colonization and invasive infection.[30] Current IDSA recommendations for prophylaxis are for selected patient groups at sufficient risk for invasive candidiasis, such as those undergoing therapy that produces prolonged neutropenia or patients receiving certain solid organ transplantations.[ 12] Preemptive therapy may be appropriate and should be considered in certain risk groups (Table 6). Breakthrough Infections
Breakthrough fungemia in patients receiving systemic antifungal therapy or prophylaxis is increasingly reported.[ 31-34] While several potential risk factors for breakthrough infections have been identified, including neutropenia, use of corticosteroids, extended use of two or more antibiotics, and ICU stay,[32,33] resistant strains are a common culprit. A randomized study compared voriconazole with liposomal AmB in patients with neutropenia and persistent fever.[20] Components of a composite score for treatment success included absence of breakthrough fungal infection, survival for 7 days after the end of therapy, absence of premature discontinuation of therapy, resolution of fever during the period of neutropenia, and successful treatment of baseline fungal infection. Voriconazole did not meet the criteria for noninferiority to liposomal AmB with respect to overall response to empiric therapy.[20] (Noninferiority was defined as a difference in success rates of no more than 10%.) However, the individual elements of the composite score for success indicated that the two treatments were similar. An ad-vantage of voriconazole treatment was that it significantly reduced breakthrough fungal infections (P = .02).[20] However, the potential exists for breakthrough infections even with the newer broad-spectrum azoles.
In one study, breakthrough fungal infections were reported for 13 of 139stem cell transplant patients who had been successfully treated with voriconazole for proven or probable invasive aspergillosis.[35] The most common cause of these breakthrough infections was Zygomycetes (46% of patients), followed by C glabrata (31% of patients). Breakthrough zygomycosis after voriconazole treatment has been reported in highly immunosuppressed patients who were being treated for graft-vs-host diseasein other transplant centers[36] and may be associated with the underlying condition.[35] However, the emergence of voriconazole resistance among C glabrata species is just beginning to be reported. This underscores the need for continued surveillance and vigilance in patients at high risk for the development of resistance. Refractory Infections
Numerous uncertainties complicate the treatment of infections that are refractory to therapy, but certain considerations will optimize the course of treatment. First, it is critical that an accurate diagnosis has been made with certainty. Failure to do so is often the primary reason for treatment failure. Second, optimizing the dosing of drugs is crucial not only for efficacy but also for prevention of resistance. Often, suboptimal doses are used, particularly with the azole compounds that are administered in low doses. Such doses are not only less effective but also may select for resistant strains. Third, the newer agents, such as caspofungin and voriconazole, showgood activity against most Candida species and hold promise as salvage agents for the treatment of invasive candidiasis. Thus, they may be good options for relapsing infections.[37,38] Fourth, combination therapy with different classes of agents is a feasible option in patients who do not respond to conventional monotherapy, as well as in clinically challenging settings, such as in patients with endocarditis, meningitis, and hepatosplenic candidiasis. Several combinations, including flucytosine-AmB, flucytosine-azole, AmB-azole, and azole-echinocandin, have been tried; however, synergy, antagonism, and indifference among antifungal agents have been reported.[39] Although still of uncertain clinical significance, the first large-scale clinical trial of combination therapy for candidemia demonstrated favorable results in non-neutropenic patients.[ 40] Fluconazole (12 mg/kg/d) plus AmB (0.7 mg/kg/d) was not antagonistic compared with fluconazole alone, and the combination trended toward more rapid clearance of yeast from the bloodstream and an overall improved success rate of 69% (77 of 112 patients) compared with 56% (60 of 107 patients) (P = .043) with fluconazole alone.[40] Finally, a possibility that warrants some discussion, particularly in patients with refractory infections, is the use of immunomodulation using growth factors and various types of cytokines. While further studies are needed, recombinant macrophage colony- stimulating factor has shown variable responses as adjunctive therapy in candidiasis.[41] The immunosuppressive drugs cyclosporine, tacrolimus, and sirolimus even exert potent antifungal effects against a variety of pathogenic fungi.[42] These compounds, which are currently indicated for immunosuppressive therapy to treat and prevent rejection of transplanted organs, may prove useful in the management of invasive fungal infections. Prevention of Resistance
First, as already mentioned, proper identification of the yeast and correct dosing are of utmost importancefor successful clinical outcomes and in the prevention of resistance. Second, adherence to basic infection control measures, such as hand-washing, is requisite. In studies reported in the literature, Candida species have been isolated from the hands of 15% to 58% of health-care workers in the ICU and other health-care settings.[43,44] In this age of resistance, it is imperative that hand-washing and other practical measures be employed to avoid exposing the patient (who is already at high risk) to potentially drug-resistant fungi. Third, when feasible, central venous catheters should be removed, preferably early in the management of candidemia, particularly in patients with catheter-related candidemia.[12,45,46] The yeasts produce biofilms that make them extremely resistant to some antifungal agents, and the antifungal activity of certain drugs varies depending on whether cells are in biofilms or planktonic forms. For example, echinocandins and lipid formulations of AmB have better activity against biofilm yeast than do azoles or AmB deoxycholate,[47] although the clinical value of this observation remains uncertain. The risk of increased morbidity and mortality associated with the removal of vascular catheters (and reinsertion of new ones) must be weighed against the risk of persistent fungemia and increased risk of seeding of target organs.[44] Finally, switching to alternative classes of antifungal drugs should alleviate some of the resistance issues. Conclusions The treatment of patients with candidemia or deep candidal infections remains a challenge despite the availability of an ever-widening choice of antifungal drugs. Underlying this challenge is the growing problem of resistant strains. Microorganisms may be innately resistant to certain agents, but-more important clinically-secondary resistance may develop during or after therapy. According to the latest figures from the CDC, about 70% of the bacterial infections acquired by nearly 2 million people in US hospitals each year are resistant toat least one drug, leading to 90,000 deaths per year.[48] While antifungal resistance has not reached such epidemic proportions, the growing population of patients at risk and the trends in the changing epidemiology of fungal infections point to the need for increased awareness of the problem and integration of clinical data, predictive resistance testing, and diligent infection control practices into therapeutic strategies. Numerous questions remain unanswered, the most important being who should receive antifungal therapy, which agent should be used, and when therapy should be initiated (keeping in mind that prompt therapy is critical to successful outcomes). Of equal importance is establishing a standard for susceptibility testing of fungal isolates. The NCCLS methodology is generally accepted as the gold standard, but the search for more practical and reliable alternatives continues, because the need for rapid identification of yeast isolates is paramount. The prevention of fungal infections in high-risk patients remains an important goal, and the institution of appropriate therapeutic and preventive measures should improve patient outcomes as well as keep clinical resistance at a level that is manageable.
Disclosures:
Dr. Perfect has acted as a consultant for Pfizer, Enzon, Merck, Fujisawa, PLIVA, and Schering-Plough.
References:
1. Hajjeh RA, Sofair AN, Harrison LH, et al: Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol 42:1519-1527, 2004.
2. Trick WE, Fridkin SK, Edwards JR, et al: National Nosocomial Infections Surveillance System Hospitals. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin Infect Dis 35:627-630, 2002.
3. McNeil MM, Nash SL, Hajjeh RA, et al: Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin Infect Dis 33:641-647, 2001.
4. Viscoli C, Girmenia C, Marinus A, et al: Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin Infect Dis 28:1071- 1079, 1999.
5. Sullivan DJ, Moran GP, Pinjon E, et al: Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candidadubliniensis and Candida albicans. FEMS Yeast Res 4:369-376, 2004.
6. Collin B, Clancy CJ, Nguyen MH: Antifungal resistance in non-albicans Candida species. Drug Resist Updat 2:9-14, 1999.
7. Nguyen MH, Peacock JE Jr, Morris AJ, et al: The changing face of candidemia: Emergence of non-Candida albicans species and antifungal resistance. Am J Med 100:617-623, 1996.
8. Krcmery V, Barnes AJ: Non-albicans Candida spp. causing fungaemia: Pathogenicity and antifungal resistance. J Hosp Infect 50:243- 260, 2002.
9. Morace G, Amato G, Bistoni F, et al: Multicenter comparative evaluation of six commercial systems and the National Committee for Clinical Laboratory Standards M27-A broth microdilution method for fluconazole susceptibility testing of Candida species. J Clin Microbiol 40:2953-2958, 2002.
10. National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards: Wayne, Pa; 1997.
11. National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards: Wayne, Pa; 2002.
12. Pappas PG, Rex JH, Sobel JD, et al: Guidelines for treatment of candidiasis. Clin Infect Dis 38:161-189, 2004.
13. Ostrosky-Zeichner L, Rex JH, Pappas PG, et al: Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother 47:3149-3154, 2003.
14. Cuenca-Estrella M, Lee-Yang W, Ciblak MA, et al: Comparative evaluation of NCCLS M27-A and EUCAST broth microdilution procedures for antifungal susceptibility testing of Candida species. Antimicrob Agents Chemother 46:3644-3647, 2002.
15. Hospenthal DR, Murray CK, Rinaldi MG: The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn Microbiol Infect Dis 48:153-160, 2004.
16. Colombo AL, Perfect J, DiNubile M, et al: Global distribution and outcomes for Candida species causing invasive candidiasis: Results from an international randomized doubleblind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis 22:470-474, 2003.
17. Pfaller MA, Diekema DJ, International Fungal Surveillance Participant Group: Twelve years of fluconazole in clinical practice: Global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect 10(suppl 1):11-23, 2004.
18. Pfaller MA, Lockhart SR, Pujol C, et al: Hospital specificity, region specificity, and fluconazole resistance of Candida albicans bloodstream isolates. J Clin Microbiol 36:1518-1529, 1998.
19. Wingard, JR: Importance of Candida species other than C albicans as pathogens in oncology patients. Clin Infect Dis 20:115-125, 1995.
20. Walsh TJ, Pappas P, Winston DJ, et al: National Institute of Allergy and Infectious Diseases Mycoses Study Group. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225-234.
21. Mora-Duarte J, Betts R, Rotstein C, et al: Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 347:2020-2029, 2002.
22. Walsh T, Sable C, Depauw B, et al: A randomized, double-blind multicenter trial of caspofungin v liposomal amphotericin B for empirical antifungal therapy of persistently febrile neutropenic patients. In: Program and abstracts of the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14-17, 2003; Chicago. Abstract M-1761.
23. Glasmacher A, Hahn C, Molitor E, et al: Fungal surveillance cultures during antifungal prophylaxis with itraconazole in neutropenic patients with acute leukaemia. Mycoses 42:395- 402, 1999.
24. Goodman JL, Winston DJ, Greenfield RA, et al: A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 326:845-851, 1992.
25. Rotstein C, Bow EJ, Laverdiere M, et al: Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis 28:331-340, 1999.
26. Bow EJ, Laverdiere M, Lussier N, et al: Antifungal prophylaxis for severely neutropenic chemotherapy recipients: A meta analysis of randomized-controlled clinical trials. Cancer 94:3230-3246, 2002.
27. Swoboda SM, Merz WG, Lipsetta PA: Candidemia: The impact of antifungal prophylaxis in a surgical intensive care unit. Surg Infect (Larchmt) 4:345-354, 2003.
28. Castagnola E, Machetti M, Bucci B, et al: Antifungal prophylaxis with azole derivatives. Clin Microbiol Infect 10(suppl 1):86-95, 2004.
29. Winston DJ, Maziarz RT, Chandrasekar PH, et al: Intravenous and oral itraconazole versus intravenous and oral fluconazole for longterm antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 138:705-713, 2003.
30. Bennett JE, Izumikawa K, Marr KA: Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother 48:1773-1777, 2004.
31. Nucci M, Colombo AL, Spector N, et al: Breakthrough candidemia in neutropenic patients. Clin Infect Dis 24:275-276, 1997.
32. Uzun O, Ascioglu S, Anaissie EJ, et al: Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin Infect Dis 32:1713-1717, 2001.
33. Nucci M, Colombo AL: Risk factors for breakthrough candidemia. Eur J Microbiol Infect Dis 21:209-211, 2002.
34. Myoken Y, Kyo T, Kohara T, et al: Breakthrough fungemia caused by azole-resistant Candida albicans in neutropenic patients with acute leukemia. Clin Infect Dis 36:1496-1497, 2003.
35. Imhof A, Balajee SA, Fredricks DN, et al: Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis 39:743-746, 2004.
36. Marty FM, Cosimi LA, Baden LR: Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stemcell transplants. N Engl J Med 350:950-952, 2004.
37. Ostrosky-Zeichner L, Oude Lashof AM, Kullberg BJ, et al: Voriconazole salvage treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis 22:651-655, 2003.
38. Johnson MD, Perfect JR: Caspofungin: First approved agent in a new class of antifungals. Expert Opin Pharmacother 4:807-823, 2003.
39. Johnson MD, MacDougall C, Ostrosky- Zeichner L, et al: Combination antifungal therapy. Antimicrob Agents Chemother 48:693- 715, 2004.
40. Rex JH, Pappas PG, Karchmer AW, et al: A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis 36:1221-1228, 2003.
41. Nemunaitis J, Shannon-Dorcy K, Appelbaum FR, et al: Long-term follow-up of patients with invasive fungal disease who received adjunctive therapy with recombinant human macrophage colony-stimulating factor. Blood 82:1422-1427, 1993.
42. Blankenship JR, Steinbach WJ, Perfect JR, et al: Teaching old drugs new tricks: Reincarnating immunosuppressants as antifungal drugs. Curr Opin Investig Drugs 4:192- 199, 2003.
43. Strausbaugh LJ, Sewell DL, Ward TT, et al: High frequency of yeast carriage on hands of hospital personnel. J Clin Microbiol 32:2299-2300, 1994.
44. Pfaller MA: Epidemiology of candidiasis. J Hosp Infect 30(suppl):329-338, 1995.
45. Walsh TJ, Rex JH: All catheter-related candidemia is not the same: Assessment of the balance between the risks and benefits of removal of vascular catheters. Clin Infect Dis 34:600-602, 2002.
46. Rex JH, Bennett JE, Sugar AM, et al: Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 21:994-996, 1995.
47. Kuhn DM, George T, Chandra J, et al: Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46:1773-1780, 2002.
48. National Institute of Allergy and Infectious Diseases Fact Sheet. The Problem of Antibiotic Resistance. Available at: http// www.niaid.nih.gov/factsheet/antimicro.htm. Accessed November 10, 2004.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.