Invasive Candida Infections: The Changing Epidemiology
Candida is recognized as the fourth most common cause of bloodstreaminfection in the United States, with a high attributable mortalityrate. While Candida albicans remains the most common pathogen, nonalbicansCandida species, including Candida glabrata and Candidakrusei, with greater resistance to triazoles are being increasingly isolated.These epidemiologic changes are attributable to a combinationof factors, such as the use of fluconazole prophylaxis, changes in patientdemographics and underlying diseases, and use of therapeuticstrategies that may pose unique risks. Of particular concern is the increasedprevalence of species that are resistant to the azole antifungals.Candida glabrata, for example, is often resistant to fluconazole,and its ability to become cross-resistant to newer azole antifungals is arecent concern. Increasing evidence underscores the need to carefullyevaluate antifungal treatment options, according to both host and therapeuticrisks for drug resistance.
Candida is recognized as the fourth most common cause of bloodstream infection in the United States, with a high attributable mortality rate. While Candida albicans remains the most common pathogen, nonalbicans Candida species, including Candida glabrata and Candida krusei, with greater resistance to triazoles are being increasingly isolated. These epidemiologic changes are attributable to a combination of factors, such as the use of fluconazole prophylaxis, changes in patient demographics and underlying diseases, and use of therapeutic strategies that may pose unique risks. Of particular concern is the increased prevalence of species that are resistant to the azole antifungals. Candida glabrata, for example, is often resistant to fluconazole, and its ability to become cross-resistant to newer azole antifungals is a recent concern. Increasing evidence underscores the need to carefully evaluate antifungal treatment options, according to both host and therapeutic risks for drug resistance.
Over the past 2 decades, population- and hospital-based surveillance data have indicated trends toward a higher incidence of fungal infections, with a broader diversity of contributing pathogens. Bloodstream infections (BSIs) are an important cause of morbidity and mortality in the United States, particularly in hospitalized patients with extended ICU stays. According to National Nosocomial Infections Surveillance (NNIS) data, there was a 5-fold increase in incidence of nosocomial fungal BSIs, from 0.1 to 0.5 per 1,000 patients discharged, between 1980 and 1990.[1] In this study, patients with fungemia were reported to be more likely to die during hospitalization than were patients with BSIs caused by nonfungal pathogens.[1] A recent large study of BSIs among US hospitalized patients confirmed that the increased frequency of fungal BSIs has been a persistent trend spanning 3 decades.[ 2] Candida species are currently the fourth most common cause of BSI in the United States. Because of advanced disseminated disease at the time of diagnosis, the frequency ofaccompanying septic shock, and the critically ill state of the host, crude mortality after candidemia is high. Decreased sensitivity of blood culture poses impediments to initiating early, organism-directed antifungal therapy. Antigen-based and molecular diagnostic methods-although improving in performance characteristics- are still evolving. There has been a continuous shift in the Candida species that cause invasive disease, from species susceptible to azole antifungals to more resistant strains, and this shift has posed a growing challenge to the medical community. Therefore, recognitionof the risk factors for invasive candidiasis and awareness of the distribution, characteristics, and susceptibility patterns of specific pathogens assume great importance in developing infection control strategies and effective pharmacotherapeutic interventions. This article will review the recent changes in the epidemiology of candidemia, including the emergence of antifungal resistance. Epidemiology of Candidemia During the 1980s, a substantial increase in the frequency of candidemia was documented; a study of more than25,000 patients admitted to US hospitals estimated that BSIs increased at least fivefold during that decade.[3] Further, a nationwide surveillance program of more than 10,000 isolates from patients with BSIs from 49 US hospitals identified Candida as the fourth most common pathogen, accounting for 8% of all infections and having the highest crude mortality rate (40%).[4] A 15-year retrospective cohort study of nosocomial candidemia estimated the attributable mortality rate to be even higher, reaching 49%.[5]
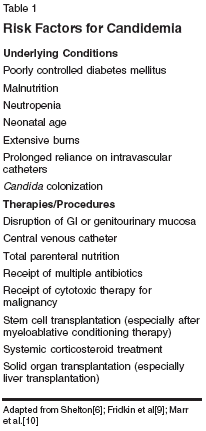
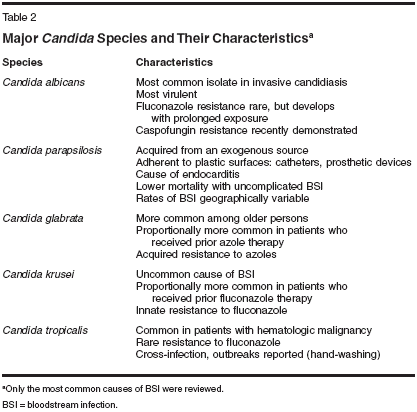
Although fungal infections have historically represented a relatively small epidemiologic concern compared with other microbial infections, a number of factors have had the combined effect of increasing the frequency of invasive infections caused by Candida species during the past 2 decades. In general, when exposure to the pathogen coincides with a weakened immune system, the situation is ripe for invasive infection and disseminated disease. Exposure to Candida species can be established by endogenous organisms bypassing normalGI barriers or, with exogenous exposure, typically through a vascular route. Classic factors that increase the risk of infection include conditions or procedures that disrupt the normal barriers provided by skin or the GI tract.[6] Abdominal surgeries, severe burns, and liver transplantation are examples of classic settings in which normal barriers are disrupted, potentially leading to infection with an otherwise harmless organism that colonizes the GI tract. Use of agents that disrupt the complex GI microbial ecology, such as broad-spectrum antibacterials, may increase risks secondary to increased GI colonization.[7,8] Patients who have both impaired GI integrity and immune deficiency, such as those receiving cytotoxic chemotherapy for hematologic malignancies or those undergoing stem cell transplantation after myeloablative conditioning therapy, are at particularly high risk for invasive candidal infection (Table 1).[6,9,10] Candida organisms may be introduced into the bloodstream by infection through a catheter or by contaminated infusate. Thus, prolonged stay in the ICU and use of total parenteral nutrition have been associated with elevated rates of nosocomial candidal infections.[11-16] Premature neonates with indwelling catheters are at particularly high risk for invasive candidiasis. The healthcare professional can also play a role in transmitting nosocomial candidal infections: A high frequency of candidal carriage on the hands of hospital personnel[17] and low compliance with hand-washing regulations have been implicated in nosocomial outbreaks of candidiasis.[18,19] Different Candida species predominate as pathogens in different patient populations, since each Candida species is unique in its ability to cause disease and exhibit inherent or acquired resistance to antifungal drugs(Table 2). Candida albicans continues to be the most common Candida isolate recovered from patients with BSIs worldwide, but infections caused by other Candida species now constitute approximately half of cases, depending on the geographic region and patient population. While the absolute frequency of different Candida species varies among institutions, based on the patient population and specific host factors, certain trends have been appreciated in large surveillance studies (Table 3).[20-27]
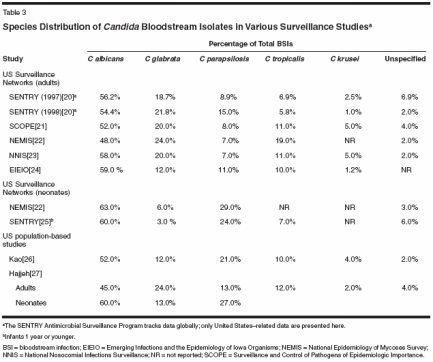
Data from the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) program for April 1995 through June 1996 showed that 48% of Candida infections were caused by non-albicans Candida, including 20% from Candidai glabrata, 11% from Candida tropicalis, 8% from Candida parapsilosis, and 5% from Candida krusei.[21] Other surveillance studies spanning 1993 to 2001 have reported similar proportions, ranging from 42% to 52% of BSIs caused by non-albicans Candida in adults with nosocomial infection in the United States.[20,22,24,25] Either C glabrata or C parapsilosis is the second most common cause of BSI, depending on the geographic locale. In the United States, approximately 20% of Candida BSIs are caused by C glabrata; C parapsilosis, C tropicalis, and C krusei were less common causes of BSIs. Similarly, in a Canadian surveillance study that compared the species causing BSI according to year (1985 vs 1996 through 1998), the proportion causedby C glabrata increased by 9% and the proportion caused by C tropicalis decreased by 7% during the latter years.[28] The proportion of BSIs caused by C albicans and C parapsilosis decreased by 10% and 4%, respectively; however, the timedependent differences in proportions were not statistically significant.[28] Several studies have reported decreased attack rates of candidemia in ICU patients[23] and in stem cell transplant recipients.[10] The overall decrease in candidemia is primarily a result of the decreased incidence of BSIs caused by C albicans. The NNIS data showed that between 1989 and 1999, the incidence of C albicans BSIs significantly decreased (P< .001). However, during the same time interval, there was a significant increase inthe incidence of BSIs caused by C glabrata (P = .05) (Figure).[23]
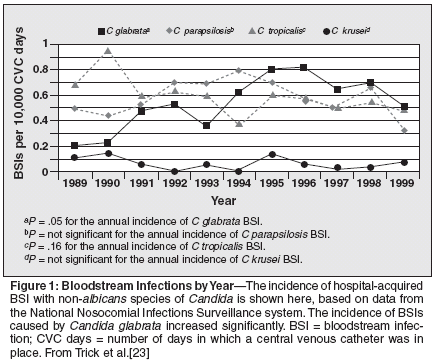
Use of fluconazole is, at least in part, responsible for both trends; however, other factors that present unique selection pressures have influenced the change in epidemiology. These factors include changes in patient demographics and underlying diseases, therapeutic strategies posing unique risks and, perhaps, differences in colonization patterns influenced by asyet- incompletely described geographic and host variables. C albicans and C parapsilosis account for greater proportions of infections in neonates, while C glabrata infections are rarely reported in this population.[22] SENTRY data for 1997 to 2000 demonstrated similar age-related differences in the distribution of Candida. The proportion of BSIs caused by C glabrata was low in patients 1 year or younger and those aged 2 to 15 years (3% each), and highest in patients 65 years or older (23%). C parapsilosis represented21% to 24% of isolates from patients in the youngest groups, compared with 12% for patients 16 years or older.[25] In contrast, results of the Emerging Infections and the Epidemiology of Iowa Organisms (EIEIO) study, which examined BSIs in patients ranging in age from younger than 1 year to older than 65 years, showed an increased frequency of C glabrata BSI with advancing age (P = .02).[24] In this study, more than 25% of BSIs in patients 65 years or older were caused by C glabrata, compared with none in patients younger than 1 year. Risks for C parapsilosis and C glabrata infections clearly differ; hence, the distribution of species according to age is likely the combined result of differing host and therapeutic selection pressures. It is also of interest that rates of C glabrata colonization increase with advancing age; whether this is the result of changes in mucosal factors influencing colonization or differences in disease distribution is not yet clear.[29] The species that cause disease also differ according to geographic region.[ 21] The SCOPE program revealed a greater proportion of BSIs caused by C albicans in the southwest region of the United States (70%)than in other regions of the country, and the greatest percentages of BSIs caused by C glabrata were observed in the southeast and northeast regions.[ 21] Similar differences are observed in international surveillance studies.[ 20,30] In general, the distribution of Candida species has been similar in the United States, Canada, and Europe; in these regions, the proportion of BSIs caused by C glabrata has increased, and BSIs caused by C parapsilosis have decreased in recent years. In Latin America and the Asian-Pacific region, C glabrata is a less frequent cause of BSI.[30] In 1997, 2.4% of BSIs in Latin America were caused by C glabrata, compared with 18.7% in the United States.[20] In 1998, the corresponding numbers were 9.2% and 21.8%, respectively. The high proportion of BSIs caused by C glabrata in certain parts of the world is in part secondary to successful prevention of C albicans infections with fluconazole prophylaxis. Non-albicans Candida species, especially C glabrata, predominate in both pediatric and adult oncology centers that use prophylactic fluconazole.[ 10,31] In a European surveillance study of adults with cancer and candidemia, C albicans was the pathogen in 70% of patients with solid tumors and only 36% of patients with hematologic malignancies.[32] Another study demonstrated that the overall attack rate and proportion of C albicans BSIs decreased in stem cell transplant recipients after the adoption of prophylactic fluconazole[10]; this change is likely secondary to decreased C albicans colonization in the GI tract as a result of fluconazole prophylaxis. Specifically, fluconazole prophylaxis is associated with effective reduction in GI colonization with C albicans and increased colonization with more resistant species, such as C glabrata.[10] The ability of C glabrata isolates to become cross-resistant to newer azole drugs, such as voriconazole,[33] is of recent concern; one study reported that C glabrata is a frequent cause of breakthrough infection in patients receiving voriconazole for other invasive fungal infections (aspergillosis).[ 34] Acquired Resistance in Candida Species Concern about antifungal resistance in Candida arose in the mid- 1990s, with reports of fluconazoleresistant C albicans causing oropharyngeal candidiasis in patients with AIDS who were receiving long-term fluconazole prophylaxis.[35,36] Detailed study of the mechanism of resistance indicated that C albicans can acquire resistance to fluconazole via mechanisms that lead to decreased accumulation of fluconazole within the cell or that otherwise reduce or block the drug's ability to interact normally with its target enzyme, lanosterol demethylase.[37,38] Such cellular changes can also confer crossresistance to other azole antifungals, such as itraconazole.[37] In some isolates, inducibility of fluconazole resistance appears to be associated with selection of a resistant clone from a heterogeneous population of cells.[39] The use of highly active antiretroviral therapy (HAART) for HIV infection greatly diminished the problem of triazole-resistant oropharyngeal candidiasis.[40] Azole resistance among bloodstream isolates of C albicans appears to be uncommon. Several investigators reported that C albicans isolates with high-degree azole resistance can cause candidemia in severely immunosuppressed patients who receive prolongedfluconazoleprophylaxis[ 10,39,41,42]; however, this is a minor problem in population studies. Hajjeh and coworkers[ 27] reported that only 1.2% of C albicans isolates were resistant to fluconazole (minimal inhibitory concentration [MIC]: 64 μg/mL or higher) and 0.9% were resistant to itraconazole (MIC: 1μg/mL or higher). It has recently become apparent that C albicans can demonstrate clinically significant resistance to echinocandin antifungals, with a mechanism that involves mutation in one of several genes encoding the target enzyme complex beta-1,3 glucan synthase.[43,44] The overall clinical significance of this problem awaits further studies. In contrast, fluconazole resistance commonly occurs in C glabrata.Among population studies, the proportion of C glabrata isolates that demonstrate high MICs to fluconazole has been as high as 27%.[45] In the SENTRY program, 8.7% of C glabrata isolates demonstrated resistance to fluconazole (MIC90: 32 μg/mL) and 36.9% were resistant to itraconazole (MIC90: 2.0 μg/mL).[46] Acquisition of fluconazole resistance is usually associated with increased expression of cellular efflux pumps; one study demonstrated that fluconazole MICs increase with cumulative exposure to the drug.[47] As mentioned above, this organism can also demonstrate resistance to the newer azoles, and organisms that have high MICs to voriconazole have emerged as a cause of breakthrough candidemia.[34] Finally, while C krusei isolates still account for a minority of BSIs worldwide, the organism is known to be innately resistant to fluconazole. It appears that the newer azole antifungals have increased activity against this organism, by virtue of better inhibition of the target enzyme.[48] The potential for C krusei acquisition of resistance to these new azoles has not yet been fully evaluated. Conclusions The incidence of invasive fungal BSIs has increased, largely propelled by advances in medical and surgical therapies that provide an optimum milieu for opportunistic infections in patients who are already immunocompromised. During the azole era, tremendous benefits were seen in curtailing C albicans BSIs; however, other, more resistant Candida species emerged as a cause of disease. This changing epidemiology has been observed in individual institutions and in population-based surveillance programs. However, the emergence of triazole- resistant Candida species must be kept in perspective; while such species are clearly on the rise, C albicans continues to account for most cases of candidemia, and the low rate of azole resistance in this species is reassuring. The potential for Candida isolate acquisition of resistance to newerazole antifungals and echinocandins has been appreciated recently, although the clinical significance of this will await increased use of these drugs and future studies. Most important, these studies demonstrate the need to identify the specific pathogen and to carefully evaluate treatment options according to both host and therapeutic risks of drug resistance. Given the increased numbers of antifungal drugs available, laboratory-directed decision making may assume a greater role in the future. However, at present, appropriate therapeutic strategies may be devised with an understanding of how each Candida species differs with respect to its pathogenic mechanism(s) and potential for resistance.
Disclosures:
Dr. Marr has received research grant support from Pfizer, Fujisawa, Merck, and Bio-Rad. She has acted as a consultant for Bio-Rad, Enzon, Schering-Plough, Fujisawa, Merck, and Pfizer.
References:
1. Jarvis WR: Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 20:1526-1530, 1995.
2. Martin GS, Mannino DM, Eaton S, et al: The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546-1554, 2003.
3. Banerjee SN, Emori TG, Culver DH, et al: Secular trends in nosocomial primary bloodstream infections in the United States, 1980- 1989. National Nosocomial Infections Surveillance System. Am J Med 91(suppl 3B):86S- 89S, 1991.
4. Edmond MB, Wallace SE, McClish DK, et al: Nosocomial bloodstream infections in United States hospitals: A three-year analysis. Clin Infect Dis 29:239-244, 1999.
5. Gudlaugsson O, Gillespie S, Lee K, et al: Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 37:1172- 1177, 2003.
6. Shelton BK: Opportunistic fungal infections in the critically ill. Crit Care Nurs Clin North Am 12:323-340, 2000.
7. Wey SB, Mori M, Pfaller MA, et al : Risk factors for hospital-acquired candidemia. A matched case-control study. Arch Intern Med 149:2349-2353, 1989.
8. Bross J, Talbot GH, Maislin G, et al: Risk factors for nosocomial candidemia: A case-control study in adults without leukemia. Am J Med 87:614-620, 1989.
9. Fridkin SK, Jarvis WR: Epidemiology of nosocomial fungal infections. Clin Microbiol Rev 9:499-511, 1996.
10. Marr KA, Seidel K, White TC, et al: Candidemia in allogeneic blood and marrow transplant recipients: Evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis 181:309-316, 2000.
11. Kam LW, Lin JD: Management of systemic candidal infections in the intensive care unit. Am J Health Syst Pharm 59:33-41, 2002.
12. Khan ZU, Chandy R, Metwali KE: Candida albicans strain carriage in patients and nursing staff of an intensive care unit: A study of morphotypes and resistotypes. Mycoses 46:479-486, 2003.
13. Jarvis WR, Edwards JR, Culver DH, et al: Nosocomial infection rates in adult and pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Am J Med 91(suppl 3B):185S-191S, 1991.
14. Leibovitz E: Neonatal candidosis: Clinical picture, management controversies and consensus, and new therapeutic options. J Antimicrob Chemother 49(suppl 1):69-73, 2002.
15. Hilfiker ML, Azizkhan RG: Mycotic infections in pediatric surgical patients. Semin Pediatr Surg 4:239-244, 1995.
16. McKinnon PS, Goff DA, Kern JW, et al: Temporal assessment of Candida risk factors in the surgical intensive care unit. Arch Surg 136:1401-1408; discussion 1409, 2001.
17. Strausbaugh LJ, Sewell DL, Ward TT, et al: High frequency of yeast carriage on hands of hospital personnel. J Clin Microbiol 32:2299-2300, 1994.
18. Bischoff WE, Reynolds TM, Sessler CN, et al: Handwashing compliance by health care workers: The impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 160:1017-1021, 2000.
19. Pittet D, Mourouga P, Perneger TV: Compliance with handwashing in a teaching hospital. Infection control program. Ann Intern Med 130:126-130, 1999.
20. Pfaller MA, Jones RN, Doern GV, et al: Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997-1998. Antimicrob Agents Chemother 44:747-751, 2000.
21. Pfaller MA, Jones RN, Messer SA, et al: National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: Frequency of occurrence and antifungal susceptibility in the SCOPE Program. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic. Diagn Microbiol Infect Dis 30:121-129, 1998.
22. Rangel-Frausto MS, Wiblin T, Blumberg HM, et al: National epidemiology of mycoses survey (NEMIS): Variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis 29:253-258, 1999.
23. Trick WE, Fridkin SK, Edwards JR, et al: Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin Infect Dis 35:627-630, 2002.
24. Diekema DJ, Messer SA, Brueggemann AB, et al: Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol 40:1298-1302, 2002.
25. Pfaller MA, Diekema DJ, Jones RN, et al: Trends in antifungal susceptibility of Candida species isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J Clin Microbiol 40:852-856, 2002.
26. Kao AS, Brandt ME, Pruitt WR, et al: The epidemiology of candidemia in two United States cities: Results of a population-based active surveillance. Clin Infect Dis 29:1164-1170, 1999.
27. Hajjeh RA, Sofair AN, Harrison LH, et al: Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol 42:1519-1527, 2004.
28. St-Germain G, Laverdiere M, Pelletier R, et al: Prevalence and antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites: results of a 2-year (1996 to 1998) multicenter surveillance study in Quebec, Canada. J Clin Microbiol 39:949- 953, 2001.
29. Lockhart SR, Joly S, Vargas K, et al: Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res 78:857-868, 1999.
30. Pfaller MA, Diekema DJ: Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect 10(suppl 1):11-23, 2004.
31. Mullen CA, Abd El-Baki H, Samir H, et al: Non-albicans Candida is the most common cause of candidemia in pediatric cancer patients. Support Care Cancer 11:321-325, 2003.
32. Viscoli C, Girmenia C, Marinus A, et al: Candidemia in cancer patients: A prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin Infect Dis 28:1071- 1079, 1999.
33. Drago M, Scaltrito MM, Morace G, GISIA-2 Group: In vitro activity of voriconazole and other antifungal agents against clinical isolates of Candida glabrata and Candida krusei. Eur J Clin Microbiol Infect Dis 23:619-624, 2004.
34. Imhof A, Balajee SA, Fredricks DN, et al: Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis 39:743-746, 2004.
35. Newman SL, Flanigan TP, Fisher A, et al: Clinically significant mucosal candidiasis resistant to fluconazole treatment in patients with AIDS. Clin Infect Dis 19:684-686, 1994.
36. Johnson EM, Warnock DW, Luker J, et al: Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother 35:103- 114, 1995.
37. White TC, Marr KA, Bowden RA: Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11:382-402, 1998.
38. Perea S, Patterson TF: Antifungal resistance in pathogenic fungi. Clin Infect Dis 35:1073-1080, 2002.
39. Marr KA, Lyons CN, Ha K, et al: Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob Agents Chemother 45:52-59, 2001.
40. Hospenthal DR, Murray CK, Rinaldi MG: The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn Microbiol Infect Dis 48:153-160, 2004.
41. Marr KA, Lyons CN, Rustad TR, et al: Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob Agents Chemother 42:2584-2589, 1998.
42. Nolte FS, Parkinson T, Falconer DJ, et al: Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother 41:196-199, 1997.
43. Kurtz MB, Abruzzo G, Flattery A, et al: Characterization of echinocandin-resistant mutants of Candida albicans: Genetic, biochemical, and virulence studies. Infect Immun 64:3244-3251, 1996.
44. Hernandez S, Lopez-Ribot JL, Najvar LK, et al: Caspofungin resistance in Candida albicans: Correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother 48:1382-1383, 2004.
45. Safdar A, Armstrong D, Cross EW, et al: Prospective epidemiologic analysis of triazoleresistant nosocomial Candida glabrata isolated from patients at a comprehensive cancer center. Int J Infect Dis 6:198-201, 2002.
46. Pfaller MA, Jones RN, Doern GV, et al: International surveillance of bloodstream infections due to Candida species: Frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. The SENTRY Participant Group. J Clin Microbiol 36:1886-1889, 1998.
47. Bennett JE, Izumikawa K, Marr KA. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother 48:1773-1777, 2004.
48. Fukuoka T, Johnston DA, Winslow CA, et al: Genetic basis for differential activities of fluconazole and voriconazole against Candida krusei. Antimicrob Agents Chemother 47:1213- 1219, 2003.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.