Astatine-211-Labeled MoAB Promising in Brain Cancer Patients
TORONTO, Canada-An initial clinical trial with a new radionuclide has shown extended survival in patients with glioblastoma multiforme, the most common primary adult central nervous system tumor, according to data presented to the 48th Annual Meeting of the Society for Nuclear Medicine (abstract 454).
TORONTO, CanadaAn initial clinical trial with a new radionuclide has shown extended survival in patients with glioblastoma multiforme, the most common primary adult central nervous system tumor, according to data presented to the 48th Annual Meeting of the Society for Nuclear Medicine (abstract 454).
The study, carried out by Michael Zalutsky, PhD, and his colleagues at Duke University Medical Center, involved a series of modifications of a technique they had previously developed to administer a monoclonal antibody, chemically linked to a radionuclide, directly into the "resection cavity" created by surgical removal of most of the primary tumor.
The monoclonal antibody, 81C6, reacts with tenascin, an extracellular matrix glycoprotein that is expressed on more than 95% of brain tumors, with the level of expression increasing with the degree of malignancy. Thus, there is reason to anticipate that the antibody would preferentially bind to brain tumor cells.
In earlier work, the Duke group found that such preferential binding of the radioactively labeled antibody was indeed observed after intravenous administration. Unfortunately, however, the degree of localization to tumors was quantitatively insufficient, with the result that excessively high therapeutic doses would have been required to effect tumor cell killing.
Given that more than 90% of these tumors progress locally, with relatively low potential for systemic metastasis, Dr. Zalutsky’s group then opted for direct injection of the antibody into the resection cavity, with the hope that the enclosed space would permit the use of lower overall levels of radiation.
In their first attempt, a phase I study of patients with recurrent glioblastoma multiforme using an iodine-131-labeled antibody, median survival was 52 weeks, a significant improvement over the 16 to 24 weeks typically associated with this condition (Bigner D et al: J Clin Oncol 16:2202-2212, 1998).
Two Improvements
The most recent work involved two significant improvements to the technique. First, the iodine-131 radionuclide was replaced by the heaviest halogen isotope, astatine-211 (At-211). Studies in animal models showed that the systemic distribution of At-211-labeled 81C6 was virtually identical with that observed in the same mice for iodine-131-labeled antibody.
The therapeutic advantages of the new isotope are considerable. "Astatine-211 is an emitter of alpha particles," Dr. Zalutsky said, "and is a much more effective killer of tumor cells than radionuclides used previously."
He explained that the alpha particles travel through only a few cells before being absorbed, and have a much higher probability of inducing double-strand breaks in DNA, so that DNA repair mechanisms of the tumor cells cannot undo the damage. In addition, he said, the limited range of the alpha particles means that there is much less risk of harming normal tissues.
The second improvement involved the use of recombinant DNA techniques to generate a chimeric human-mouse form of the monoclonal antibody, in place of the mouse form used previously.
"We found the chimeric form of the antibody to be more stable than the mouse form," Dr. Zalutsky said, "and consequently the residence time in the resection cavity is longer."
The presence of a human constant region in the antibody molecule also results in the generation of far fewer reactive antibodies by the host immune system, he said. This reduces the risk that the patient’s own immune response will lead to altered tissue distribution of the therapeutic agent.
"This diminished immunogenicity of the chimeric form of antibody 81C6 may prove of significant benefit in future work with patients who require repeated treatments," Dr. Zalutsky commented.
Study Patients
Patients who were selected for the study suffered from recurrent malignant supratentorial glioma that was unifocal and without ventricular access. After the resection, a flow study with a technetium-99m-labeled probe was performed to confirm the presence of a sealed resection cavity.
The radiation doses of the injected At-211-labeled antibody ranged from 2 to 10 mCi, an order of magnitude lower than those employed in the earlier I-131 studies.
Brain and body scans, together with blood analyses, revealed that more than 99% of the At-211 decay occurred within the tumor resection cavity, giving not only a more effective attack on the tumor but also reduced systemic radiotoxicity.
Only 2 of the 11 patients showed toxic effects after the treatment, with the associated neural deficits appearing 11 and 52 weeks post-treatment, respectively.
"Given the small sample size, it is unclear as to whether these effects resulted from the treatment itself or from recurrence of the tumor," Dr. Zalutsky said. "At present, the maximum-tolerated dose of At-211 remains undefined."
Even at the doses employed in this study, however, the survival times are especially encouraging). Median survival was 60 weeks, with 7 of the 11 patients surviving for more than 1 year, and with 3 still alive at 152, 164, and 174 weeks post-treatment.
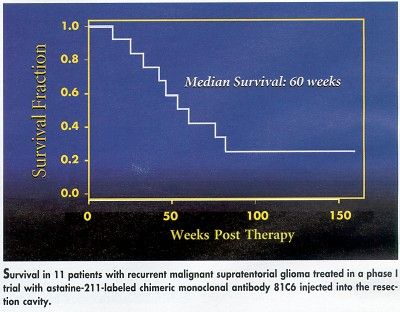
It is of particular interest that the technique appears to be readily applicable to patients as an adjunct to current approaches, Dr. Zalutsky said. Since surgical reduction of tumors is commonly performed for these patients anyway, local injection of At-211-labeled antibodies such as 81C6, or others yet to be developed, is a relatively simple addition to the procedure.