Atrial Fibrillation: Considerations for the Use of BTK Inhibitors
In this article, we describe the considerations for the use of BTK inhibitors in lymphoid malignancies from the perspective of an oncology pharmacist.
Oncology (Williston Park). 32(11):574-5.

Kelly Valla, PharmD, BCOP

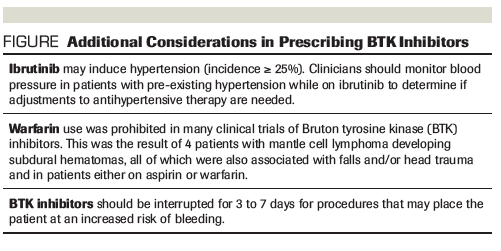
Figure. Additional Considerations in Prescribing BTK Inhibitors
Introduction
Bruton tyrosine kinase (BTK) inhibitors are a class of anticancer agents that have recently helped change the landscape of available therapies for lymphoid malignancies. Ibrutinib, the first-in-class oral BTK inhibitor, is approved by the US Food and Drug Administration for chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), Waldenstrom macroglobulinemia (WM), and chronic graft-vs-host disease (GvHD) following allogeneic stem cell transplant.[1] Acalabrutinib (Calquence), currently approved for only MCL, is also of clinical interest due to its specificity for BTK, pharmacodynamic properties in limit resistance, and favorable adverse effect profile.[2,3] Of specific concern with both of these agents, however, are cardiac adverse effects, including hypertension, atrial fibrillation, and an increased propensity for bleeding.
Association Between BTK Inhibitors and Atrial Fibrillation
In the pivotal RESONATE and RESONATE-2 trials of ibrutinib in CLL, atrial fibrillation was noted in only 3% of relapsed/refractory patients and 6% of patients treated in the frontline setting.[4,5] The PCYC-1104-CA trial found a similar rate of atrial fibrillation (4.5%) among MCL patients treated with ibrutinib.[6] Unfortunately, as the data have matured, atrial fibrillation has proven more prevalent than the signal during the follow-up seen in the registration trials. An update to the RESONATE-2 trial revealed a 10% incidence of ibrutinib-associated atrial fibrillation at a median follow-up of 29 months; of these events, only 4% were grade 3. Symptom resolution occurred in 64% of patients (complete resolution, 57%; partial resolution, 7%) and was relatively quick (median, 3 days).[7]
Mechanism of action
Atrial fibrillation occurs as a result of a reduction in PI3K-AKT pathway signaling. Inhibition of the alpha isoform of PI3K is thought to occur due to crosstalk between the BTK and PI3K-AKT pathways, or simply as a direct off-target effect. This mechanism has been validated by animal cardiac myocyte models, which found that ibrutinib administration was associated with an inhibition of PI3K-AKT signaling, leading to prolonged and abnormal action potentials within cardiac tissue.[8,9]
Acalabrutinib is considered to be an attractive alternative to ibrutinib, at least in part due to its enhanced specificity for BTK, which thereby reduces the potential for off-target effects.[10] This is supported by results of ACE-LY-004, a phase II, open-label registration study of acalabrutinib in MCL, which found no reported cases of atrial fibrillation among 124 patients at a median follow-up of 15.2 months. Among 612 patients across multiple studies, only 3% developed either atrial fibrillation or flutter.[2] However, given the late-onset atrial fibrillation seen with a longer follow-up in individuals receiving ibrutinib, similar precautions should be taken for patients on acalabrutinib until long-term safety data are available.
Comorbidities and risk factors
CLL typically occurs in older individuals who may have existing cardiac comorbidities. However, clinicians should note that a history of atrial fibrillation or other cardiac issues does not necessarily serve as an absolute contraindication to either BTK inhibitor. Risk mitigation strategies, including identification and correction of modifiable risk factors, such as obesity, smoking, alcohol consumption, high blood pressure, uncontrolled diabetes, and any existing heart disease, should be utilized in patients with pre-existing atrial fibrillation; a robust cardiac workup and optimization of medications should also be completed.[11-14] In addition, a thorough medication history is crucial in managing potential drug interactions. Both ibrutinib and acalabrutinib rely on metabolism via cytochrome P450 enzyme 3A4 (CYP3A4), and the concentration of these agents increases when administered in conjunction with other CYP3A4 inhibitors, such as azole antifungals (eg, fluconazole, posaconazole), diltiazem, verapamil, amiodarone, and/or citrus-based food or drinks (eg, grapefruit juice, Seville oranges). Interrupting and/or reducing the dose of ibrutinib has not been shown to hasten resolution, but is warranted in hemodynamically unstable patients.[13,14] Published clinical experiences and algorithms are available to assist providers in clinical decision making.
The risk of bleeding further complicates the use of BTK inhibitors in patients who develop atrial fibrillation and are at an increased risk for stroke, based on the widely accepted CHA2DS2-VASc predictive model.[13,14] Like atrial fibrillation, a higher risk of bleeding has more often been associated with ibrutinib than acalabrutinib. Approximately 50% of patients receiving ibrutinib reported some degree of bruising or bleeding; however, rates of grade 3 or higher bleeding have been reported in less than 10% of patients, primarily as a result of direct platelet dysfunction.[11,15-17] Although platelet dysfunction is not observed with acalabrutinib, bleeding and bruising have also been reported with this agent.[2,15] The decision to use anticoagulants and antiplatelet agents must be balanced with this risk; a low-molecular-weight heparin or a direct oral anticoagulant is typically preferred over warfarin due to concerns of a small number of subdural hematomas occurring in early clinical trials of ibrutinib.
Clinical uneasiness surrounding these adverse effects in patients who are otherwise eligible to receive BTK inhibitors is understandable. Performing a thorough evaluation of cardiac risk factors and a review of all concomitant medications, including herbal supplements, is crucial in order to ensure safe prescribing. Patient education should include ways to recognize symptoms and reduce modifiable risks, including avoidance of medications, as well as food and drinks, known to inhibit CYP3A4. If avoiding interacting agents is not possible, a reduction in the BTK inhibitor dose may be indicated. See the Figure for additional considerations when prescribing BTK inhibitors.
While longer follow-up is needed to confirm whether these troubling adverse effects are less common with acalabrutinib, providers may consider this agent to be a safer option for patients with MCL. The ongoing ELEVATE study, a head-to-head noninferiority trial of ibrutinib vs acalabrutinib in CLL patients, may shed more light on the toxicity differences between these agents. Attention should also be paid to BTK inhibitors still in development, including zanubrutinib (BGB-3111) and ONO/GS-4059, particularly regarding their cardiac safety. Nevertheless, should patients taking a BTK inhibitor develop atrial fibrillation, emerging evidence has shown that it is manageable, and multidisciplinary care from both a hematologist and cardiologist is recommended.
Financial Disclosure:The author has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Ibrutinib (Imbruvica) prescribing information. https://www.imbruvica.com/docs/librariesprovider7/default-document-library/prescribing-information.pdf. Accessed October 22, 2018.
2. Acalabrutinib (Calquence) prescribing information. https://www.azpicentral.com/calquence/calquence.pdf. Accessed October 22, 2018.
3. Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323-32.
4. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia. N Engl J Med. 2014;371:213-23.
5. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425-37.
6. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369;507-16.
7. Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103:1502-10.
8. McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829-30.
9. Yang T, Moslehi JJ, Roden DM. Proarrhythmic effects of ibrutinib, a clinically approved inhibitor of Bruton’s tyrosine kinase (BTK) used in cancer therapy. Circulation. 2015;132:A14587.
10. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659-67.
11. Tang CPS, McMullen J, Tam C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. 2018;59:1554-64.
12. Khalid S, Yasar S, Khalid A, et al. Management of atrial fibrillation in patients on ibrutinib: a Cleveland Clinic experience. Cureus. 2018;10:e2701.
13. López-Fernández T, Canales M, Farmakis D, et al. Ibrutinib-associated atrial fibrillation: a practical approach. Ann Hematol Oncol. 2018;5:1203.
14. de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102:1629-39.
15. Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1:2610-23.
16. Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-na¨Ä±ve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497-2506
17. Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739-45.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.
