The Bifunctional Role of Steroid Hormones: Implications for Therapy in Prostate Cancer
This review examines the relevance of a translational framework for studying therapeutic androgens in prostate cancer.
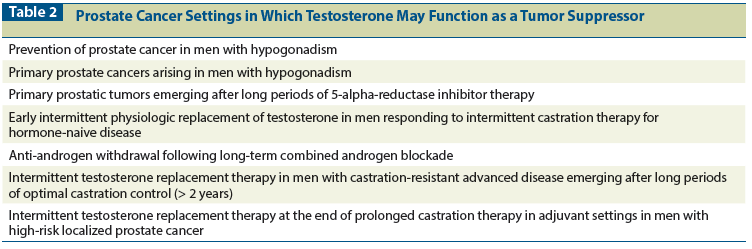
Table 1: Sex Steroids as Tumor Suppressors
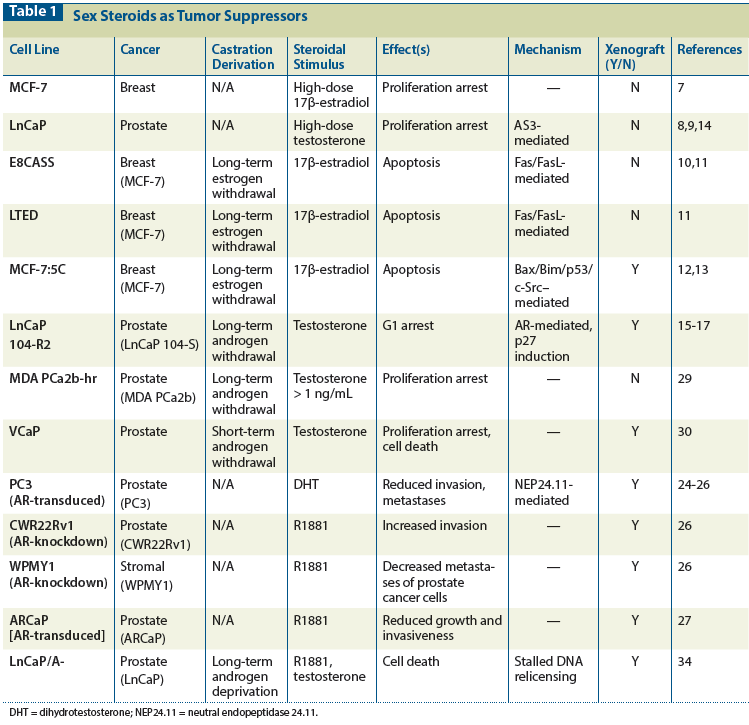
Table 2: Prostate Cancer Settings in Which Testosterone May Function as a Tumor Suppressor
Ablation of the androgen-signaling axis is currently a dominant theme in developmental therapeutics in prostate cancer. Highly potent inhibitors of androgen biosynthesis and androgen receptor (AR) function have formally improved survival in castration-resistant metastatic disease. Resistance to androgen-ablative strategies arises through diverse mechanisms. Strategies to preserve and extend the success of hormonal therapy while mitigating the emergence of resistance have long been of interest. In preclinical models, intermittent hormonal ablative strategies delay the emergence of resistant stem-cell–driven phenotypes, but clinical studies in hormone-naive disease have not observed more than noninferiority over continual androgen ablation. In castration-resistant disease, response and improvement in subjective quality of life with therapeutic testosterone has been observed, but so too has symptomatic and life-threatening disease acceleration. The multifunctional and paradoxical role of steroid hormones in regulating proliferation and differentiation, as well as cell death, requires deeper understanding. The lack of molecular biomarkers that predict the outcome of hormone supplementation in a particular clinical context remains an obstacle to individualized therapy. Biphasic patterns of response to hormones are identifiable in vitro, and endocrine-regulated neoplasms that proliferate after prolonged periods of hormone deprivation appear preferentially sex steroid–suppressible. This review examines the relevance of a translational framework for studying therapeutic androgens in prostate cancer.
Introduction
In a biomarker-driven study reported in 1941, Drs. Huggins and Hodges of the University of Chicago demonstrated reduction in elevated levels of serum acid phosphatase in five men with metastatic prostate cancer treated with estrogens and orchiectomy, whereas three men who received testosterone injections after orchiectomy exhibited increased serum levels of the enzyme.[1] Hitherto, serum elevations of acid phosphatase had been associated strictly with prostate cancer,[2] and Huggins and Hodges thus concluded that androgens activated prostate cancer. Nevertheless, in the years that followed, several investigators experimented with testosterone injections in prostate cancer. Pearson[3] of the Sloan-Kettering Institute reviewed the inconsistent biochemical and clinical responses to testosterone injections associated with these studies and puzzled over two case studies of his own, one of a hormone-naive patient, another of a castration-resistant patient, both of whom had responded to testosterone injection: “These observations invite the development of new concepts to explain the response of these prostatic cancers to alterations in the endocrine environment.”
Prout[4] also described varying outcomes in castration-resistant patients who received testosterone injection. One patient in particular, described as feeble and moribund, with extensive metastatic disease after a 5-year period of prior control with orchiectomy, had a dramatic clinical improvement following testosterone injections, going from “unable to sit without assistance” to “dancing weekly,” with sharp declines in pain and serum acid phosphatase level, and improvement in hematologic parameters. Less well emphasized in the report, however, were four patients who experienced rapid clinical deterioration following testosterone injection, including severe pain flares, development of a vesicourethral fistula, and an early death 3 days after injection. Evolution of a castration-resistant phenotype to a more androgen-sensitive condition was speculated to be an explanation for the favorable response observed. Fowler and Whitmore, on the other hand, reviewed the outcomes of 52 men with prostate cancer who received testosterone; they described a high rate of unfavorable responses in hormone-resistant disease in particular (94%) compared with treatment-naive patients (25%). Although seven patients had symptomatic benefit, no objective tumor responses were noted.[5]
That a majority of men with castration-resistant disease experience unfavorable outcomes with androgen therapy appeared to be confirmed in a randomized study of androgen priming followed by chemotherapy.[6] The goal of this study was to synchronize cells into cell-cycle proliferation with oral fluoxymesterone for 3 days prior to chemotherapy with cyclophosphamide, doxorubicin, and fluorouracil. Lack of evidence of improved response, rapid disease progression, neurological complications, and a large number of inevaluable patients in the stimulation arm, together with inferior survival outcomes, resulted in an end to this line of investigation. Maintenance of castration therapy was adopted as a recommended standard of care in castration-resistant disease. Prudence would dictate, it would seem, avoidance of all androgenic influence where possible.
However, with time, as a result of frustration with the apparent limits of efficacy of hormonal therapy, interest in testosterone replacement therapy for prostate cancer resurfaced in the clinical arena. Efforts along these lines were spurred primarily by a widening range of preclinical models that demonstrated a suppressor role for steroids in hormone-deprived settings, as well as a lower rate of transition to castration resistance with intermittent androgen suppression therapy.
Preclinical Models That Demonstrate a Bifunctional Role of Steroids
Contextual suppressive action
In MCF-7 breast[7] and LnCaP prostate cancer cell lines,[8,9] biphasic response to estrogen and testosterone, respectively, has been observed in vitro. In lower doses, the steroid hormone induces proliferation, and in high physiologic doses, cell proliferation is suppressed, raising the possibility that high physiologic doses may have therapeutic value.
When MCF-7 lines are subjected to prolonged estrogen withdrawal, proliferative variants are generated that undergo apoptosis, through varying mechanisms, on exposure to 17β-estradiol.[10-13] LnCaP cells that are placed in long-term (8 to 20 months) androgen-deprived cell culture conditions similarly acquire an androgen-repressible proliferative phenotype in vitro and in vivo.[14-17] Selected examples of the tumor suppressor actions of sex steroids in breast and prostate cancer cell lines and xenografts[8-30] are presented in Table 1.
MCF-7 and LnCaP cell lines have the capacity to revert to estrogen- and androgen-stimulated phenotypes when re-exposed to steroids over time, suggesting that these adaptive changes are epigenetic rather than a stable mutational event.[18] Not all MCF-7–derived proliferative variants in estrogen-free media demonstrate an apoptotic response to estrogen. Thus, heterogeneity in the probability of steroid-mediated suppressibility must be assumed in human disease.[18] The apoptotic effects of estrogen were observed in tumors resistant to tamoxifen and raloxifene (selective estrogen receptor modulators) as well,[19-21] arousing interest in exploiting the phenomenon of estrogen-induced apoptosis in breast cancer via a fuller mechanistic understanding.[21-23]
The tumor-suppressive effects of androgen signaling, when introduced into AR-negative prostate cancer cells, have been observed.[24,25] The critical role of the AR in the interplay of neoplastic epithelium and stroma cells has also been evaluated. Recombination of stromal WPMY1 cells with epithelial PC3 prostate cancer cells demonstrated that restoration of the AR in PC3 cells or knockdown of the AR in stromal WPMY1 cells suppressed metastases.[26] Similarly, transfection of the AR into AR-low ARCaP cells reduced growth, invasion, and migratory behavior in vitro and tumor growth in vivo.[27,28] Loss of epithelial AR in a transgenic adenocarcinoma of mouse prostate (TRAMP) model resulted in a loss of luminal cells and an increase in CK5/CK8+ basal-intermediate-like cells expressing CD44,[26] which has been associated with tumorigenic and metastatic behavior.
The contrasting effects of the AR on luminal and basal epithelium and stroma add to the complexity of modeling outcomes of hormonal manipulation, but such recombinant models may point the way forward in translational research.
Splice variants of the AR with gain-of-function properties have emerged as resistance pathways in diverse preclinical models of androgen deprivation.[31,32] Suppression or elimination of splice variants with testosterone therapy has been observed,[32] suggesting a potential therapeutic role if specific splice variant–driven disease states are broadly vulnerable to cyclic androgen therapy.
Extending the paradigm of the bifunctional effects of androgen on prostate cancer cells, it has been hypothesized that the AR functions as a DNA licensing factor, regulating origin-of-replication sites in association with other known licensing proteins with which it complexes.[33] Cells are allowed only one origin-of-replication site firing per cell cycle. In order for progression to a subsequent cell cycle to occur, degradation of licensing factors (eg, via ubiquitination and proteasomal degradation) is required. In androgen-sensitive LNCaP, CWR22Rv1, and LAPC-4 human prostate cancer cells, the AR is degraded in mitosis, whereas in nonmalignant, AR-expressing normal human prostate stromal cells in which the AR functions as a transcription factor and not as a licensing factor for DNA replication, expression of the AR remains constant throughout the cell cycle.[33]
However, the adaptive upregulation of expression of the AR in androgen-deprived conditions presents vulnerability. In LNCaP cells grown in androgen-deprived conditions, marked upregulation of the AR is observed. In these cells, as hypothesized, acute exposure to supraphysiologic doses of androgen results in stabilization of the AR with respect to origin-of-replication complexes, and this persists through mitosis through failure of sufficient AR degradation, triggering a threefold increase in cell death in the subsequent S-phase, since DNA replication cannot proceed without relicensing.[34] Similar results have been observed with xenografted cells. Castration-resistant cells that downregulate the AR on exposure to testosterone can escape cell death but may become vulnerable to re-exposure to androgen-depleted conditions.
This paradigm is the basis for the bipolar androgen therapy concept that is currently being explored in an actively recruiting clinical trial (ClinicalTrials.gov ID: NCT01750398). Whether this mechanism of cell death contributes to the suppressive effects of androgen in other experimental settings involving long-term androgen-deprived conditions is unknown, but the majority of the models are reported to demonstrate growth arrest rather than cell death.
Intermittent hormonal therapy as a paradigm for androgen-mediated suppression of disease progression
Noble, in his early work on mammary fibroadenomata, observed that hormone manipulation led to reduced tumor growth-but he noted that a more malignant and sarcomatous change emerged; he concluded that “benignancy would seem assured by the maintenance of cellular environmental conditions which allow progressive cellular growth.”[35]
In review, Foulds concluded that “there is a disturbing possibility that therapy by suppressing or retarding growth may favor progression from the responsive to the irresponsive independent state.”[36] In male rats bearing mammary tumor transplants, removal of estrogen pellets and substitution of those of lesser estrone content reduced both the regression of tumors as well as their progression to autonomy.[35] Interestingly, Noble also observed that while a primary transplant of adrenal carcinoma showed regression following removal of estrogen pellets, liver metastases from this tumor grew independently. However, transplants of the liver metastases were subsequently regulated by estrogen, suggesting that specific stromal microenvironments can significantly modify response to hormonal manipulation.
The Shionogi androgen-dependent mouse mammary carcinoma implanted in male DD/S strain mice grows quickly and regresses significantly with castration, losing 50% of its mass within 72 hours. Castration-resistant growth emerges after 40 days, culminating in a state of rapid proliferation, with complete absence of detectable nuclear AR, suggestive of androgen-independent growth.
Limiting-dilution assays of recurrent tumor cells demonstrate enrichment of androgen-independent stem cells.[37] Serial transplantation of regressing castration-sensitive tumors into intact male mice served as intermittent androgen exposure; when tumors grew, these new hosts were castrated and regressing tumors were then transplanted into a fresh intact host. In this manner, intermittent androgen suppression resulted in a threefold increase in time to androgen independence.[38]
Similar findings were obtained with intermittent androgen suppression in the LNCaP model in which mice bearing LNCaP xenografts were surgically castrated and then implanted with testosterone pellets on differing schedules (intermittent androgen suppression), while others received no replacement (continuous androgen suppression). A striking three- to fourfold delay in time to androgen-independent prostate-specific antigen (PSA) progression was noted with the intermittent schedules of parenteral testosterone replacement.[39] These preclinical models influenced translation of intermittent hormonal ablation to the clinical setting.
Clinical Observations
The preceding preclinical observations exemplify the range of experimental data that demonstrate the ability of therapeutic androgens to control prostate cancer in certain contexts. The advent of serum PSA testing, serum testosterone and dihydrotestosterone assays, radionuclide bone scans, computerized tomography, magnetic resonance imaging, and positron emission tomography (PET), and now circulating tumor cell assays, has changed the experimental landscape in prostate cancer in recent years. These advances allow for more careful assessment of disease risk, and monitoring of markers of disease progression and response to androgen therapy.
An early study in this modern era explored a monthly androgen cycling concept in hormone-naive prostate cancer patients with rising PSA levels or metastatic disease. The hypothesis of the study was that castration therapy (luteinizing hormone–releasing hormone [LHRH] agonist monthly) would induce cell kill, and that surviving cells would then be forced into cell cycle, with testosterone therapy [testosterone gel 1%, 5 g topically daily for 7 days] to increase their vulnerability to apoptotic death with subsequent castration. Mean trough testosterone levels were in the castration range, and following repletion were in the normal physiologic range. Declines in PSA levels were described as comparable to those expected with continuous hormonal ablation, with no evidence of radiographic or clinical progression. Given the small sample size (N = 36) and uncontrolled nature of the study, no insights into prolongation of time to castration resistance with this strategy were afforded.[40]
To explore the effects of testosterone replacement in castration-resistant metastatic disease in patients who had received at least 1 year of prior castration therapy, Morris et al[41] used transdermal testosterone and topical gel in 12 patients divided into 3 different cohorts (1 week duration, 4 weeks, or until progression) to assess safety and initial response to doses designed to push serum testosterone quickly into the high physiologic range. All patients achieved physiologic testosterone levels. One patient with back pain was found to have a spinal cord compression and was treated successfully with radiation therapy. Otherwise, no events indicative of symptomatic progression were noted. Discordance between minor declines in PSA level and radiologic progression was observed. In one patient, PSA level declined by 50% (he had radiologically stable disease). There were no objective responses, no improvements on PET scanning were noted, and bone scans demonstrated increased lesions in all patients in cohort 2. In cohort 3, 75% of patients demonstrated biochemical or radiologic progression.
In a second study of 15 patients with castration-resistant prostate cancer, no visceral disease, and minimal bony disease, three random doses of daily transdermal testosterone were assigned-2.5 mg, 5.0 mg, and 7.5 mg-with continued medical castration.[42] Physiologic levels of serum testosterone were not reliably achieved with this strategy. Minor declines in PSA level were seen in three patients, an initial rise and then fall in PSA level was seen in two patients, and (with one exception) no patient exhibited symptomatic progression.
In these two testosterone replacement trials in castration-resistant prostate cancer, long-term androgen deprivation control prior to the emergence of castration resistance, as exemplified in the preclinical models, was not required as part of eligibility. By contrast, a case of a man with progressive castration-resistant prostate cancer and extremely prolonged prior castration control (15 years) was reported; this patient received intramuscular testosterone replacement therapy every 2 weeks, which was designed to achieve physiologic levels of testosterone.[43] He had retroperitoneal nodal metastases but no visceral or bone metastasis. His PSA level rose sharply with the first series of intramuscular testosterone injections delivered every 2 weeks but then dropped with subsequent injections to a nadir at 18 months, with stability of nodal disease. This was accompanied by a significant improvement in a range of factors typically affected by androgen deprivation, including libido, intellectual function, and muscle strength. With continued therapy, the PSA level rose slowly over the next 8 months and then accelerated at 26 months, with progression of nodal disease. Discontinuation of testosterone replacement led promptly to castration levels of testosterone, a drop in the PSA to baseline levels, and regression of nodal metastases. Subsequent rises in PSA were not convincingly suppressed with testosterone, and the treatment was discontinued. This is one of the longest documented responses to testosterone replacement in castration-resistant metastatic prostate cancer in the literature, and the question must be asked: Did the extraordinarily long period of prior castration control of the disease allow for this degree of sensitivity to acute testosterone replacement? In a manner analogous to what was observed in the preclinical models, the duration of prior castration control may be a key predictor of efficacy. Transition to an androgen-dependent progression is similar to that seen in the preclinical models. It has been suggested that a prospective clinical trial in men with progressive castration-resistant prostate cancer and documented long periods of optimal castration control be considered to assess survival and quality-of-life outcomes.
A series of randomized phase III trials comparing continuous vs intermittent hormonal ablation in close to 5,000 men were recently reviewed.[44] It is clear from this overview that, unlike in the preclinical models, there is no evidence that intermittent hormonal ablation improves time to castration resistance-and at best, intermittent hormonal ablation is noninferior to continuous hormonal ablation. Patients spend more time on hormonal ablation than off it, and for that reason quality-of-life improvements are hard to detect.
At this juncture, it is worth drawing attention to the potentially important differences between the preclinical experiments and clinical trials. In the preclinical models, the exposure of castration-induced regressing tumors to testosterone was restored acutely either by serial transplantation of tumors from castrate to intact murine hosts or by parenteral injection of testosterone pellets. In the human setting, tumors subjected to castration therapy have been required to await the slow and variable recovery of endogenous testosterone following interruption of LHRH agonist therapy. This represents a very different form of androgen suppression strategy. Castration-resistant VCaP cells that were treated with sequentially higher doses of testosterone had less impressive apoptotic responses to androgen withdrawal than those seen in VCaP cells exposed to parenteral testosterone “boost.”[30]
It is therefore conceivable that acute replacement of testosterone in castrate men with castration-responsive tumors followed by repeat castration at clinical progression would correspond better to the preclinical models that consistently showed a threefold increase in the time to castration resistance. Such a strategy might also allow for more obvious improvement in quality-of-life outcomes, since the duration of castration would be shortened. Another difference may also be worth noting. The preclinical models of intermittent hormonal suppression tested outcomes in subcutaneous xenografts. Such experiments have not been conducted in models of bone metastases, which perhaps would be more representative of the clinical phenotype of advanced disease in which the majority of clinical trials have been conducted.[44] Noble demonstrated that it is likely that the specific stroma of a metastatic site can diverge significantly from that of a primary implant, and as a result can generate mechanisms of resistance to hormone deprivation.[35]
Another group of men represent a formal testing ground for the therapeutic value of testosterone replacement. Aging populations, obesity, and a rise in the incidence of diabetes may be contributing to an increase in primary hypogonadism among men.[45] The risk of prostate cancer with testosterone therapy in men with primary hypogonadism has been examined in multiple studies, and no convincing increase in the rate of detected cancers has emerged.[46,47] Furthermore, in a small pilot study, Morgantaler has demonstrated that men (N = 13) with newly diagnosed low-grade prostate cancer (Gleason 6, n = 12; Gleason 7, n = 1) treated with testosterone replacement for hypogonadism and undergoing active surveillance did not exhibit adverse disease progression.[48] All men underwent at least 1 rebiopsy, and of 26 follow-up biopsies, 54% were negative. Among men with prior treatment for prostate cancer who received testosterone supplementation for hypogonadism, no higher risk of relapse has been suggested. It may be hypothesized that, as in the preclinical models, a fraction of tumors arising in men with primary hypogonadism may be preferentially androgen-suppressible; however, evidence of this has yet to emerge.[49] Based on review of these data with testosterone therapy in men at risk for or with a history of prostate cancer or established low-risk disease, Morgantaler and Khera have challenged the contraindication of testosterone supplementation in these disease settings.[50,51] Maintenance of active surveillance registries of patients with newly diagnosed low-risk prostate cancer who have been placed on testosterone supplementation for symptomatic hypogonadism would be a source of useful information, while at the same time providing a necessary safeguard. Rates of progression of disease with testosterone supplementation in men on active surveillance vs rates in eugonadal men with similar risk features, also on active surveillance, would be valuable.
Other disease states in which testosterone may function as a suppressor may be hypothesized (Table 2). Men with longer periods of prior castration control, who more often exhibit response in the anti-androgen–withdrawal setting,[52] may also represent an androgen-suppressible phenotype, since anti-androgen–binding mutations of the AR may not occur at sufficient frequency to account for this response. Whether a fraction of primary tumors that arise in the setting of long-term uninterrupted finasteride or dutasteride (5-alpha reductase inhibitor) therapy may regress with drug withdrawal and subsequent re-exposure to the effects of endogenous androgen is as yet unknown. In the setting of high-risk localized disease being treated adjuvantly, men who receive prolonged castration therapy (> 2 years) following radiation or surgery may discontinue LHRH agonist therapy and await the variable recovery of gonadal testosterone over time. If the bipolar androgen therapy concept[34] is valid, acute intermittent replacement of testosterone at the end of the prolonged castration period may have a purging effect on residual prostate cancer cells and allow for swifter recovery from androgen deprivation symptoms. An intermittent testosterone replacement approach in this setting would allow for additional therapeutic bipolar cycles and facilitate recovery of endogenous pituitary function.
Conclusion
Over the last 7 decades, it has been widely believed that testosterone therapy in unselected castration-resistant metastatic disease settings is more frequently associated with disease progression than with high-quality sustained clinical responses. Nevertheless, a range of preclinical models has defined the potential value of androgens in specific contexts. Long-term androgen deprivation may predict a higher probability of response to androgens following late escape, although arguably this concept has not been optimally implemented in clinical trial designs to date. Indeed, the longest report of testosterone control of castration-resistant prostate cancer was associated with a 15-year period of prior castration control of low-volume metastatic disease. Less than 30% of hormone-naive patients with metastatic disease maintain castration control beyond 2 years, and even fewer for more than 4 years. Multicenter participation will be required to identify and accrue such patients to testosterone replacement studies, yet it is these patients who are also more likely to benefit from the quality-of-life improvements that come with relief of long-standing symptoms of androgen deprivation. Unfortunately, what distinguishes patients with short periods of castration control from those with long periods of control is as yet uncertain, and this contributes further to difficulties in defining a molecular phenotype that can predict the effect of testosterone replacement in any clinical context.
As was suggested earlier, the design of intermittent hormone ablation studies may not have strictly reproduced the design of preclinical models that allowed for a delay in the emergence of castration resistance. In hormone-naive disease, the concept of cycling castration therapy with androgen supplementation is worthy of examination, using as endpoints time to transition to castration resistance and quality of life.
Greater insights into the androgen-regulated complexities of epithelial-stromal homeostasis, including in stem cell phenotypes[53-55] in tissue-specific metastatic disease, are required; this represents the next research frontier. A role for emerging selective androgen-receptor modulators[56,57] that have differential effects on prostate, bone, and muscle tissue may become evident. The AR will continue to have a central role in these considerations[58] as the war on the androgen-signaling axis continues.
As early observers in the field noted,[3,23] much still remains to be learned. Responses to diethylstilboestrol in postmenopausal women with breast cancer were directly correlated to the time since menopause, although these were inferior when bone or visceral metastatic disease was present.[59] As we look forward, the lessons of the past cannot be forgotten.
Financial Disclosure:The author has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Huggins C, Hodges CV. Studies on prostate cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-7.
2. Gutman AB, Gutman EB. An “acid” phosphatase occurring in the serum of patients with metastasizing carcinoma of the prostate gland. J Clin Invest. 1938:17:473-8.
3. Pearson OH. Discussion of Dr Huggins’ paper “control of cancers of man by endocrinological methods.” Cancer Res. 1957;17:473-9.
4. Prout GR Jr, Brewer WR. Response of men with advanced prostatic carcinoma to exogenous administration of testosterone. Cancer. 1967;20:1871-8.
5. Fowler JE Jr, Whitmore WF Jr. The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J Urol. 1981;126:372-5.
6. Manni A, Bartholomew M, Caplan R, et al. Androgen priming and chemotherapy in advanced prostate cancer: evaluation of determinants of clinical outcome. J Clin Oncol. 1988;6:1456-66.
7. Soto AM, Sonnenschein C. Cell proliferation of estrogen-sensitive cells: the case for negative control. Endocr Rev. 1987;8:44-52.
8. Sonnenschein C, Olea N, Pasanen ME, Soto AM. Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res. 1989;49:3473-81.
9. Launoit Y, Veilleux R, Dufour M, et al. Characteristics of the biphasic action of androgens and of the potent antiproliferative effects of the new pure anti-estrogen EM-139 on cell cycle kinetic parameters in LNCaP human prostatic cancer cells. Cancer Res. 1991;51:5165-70.
10. Lee Y, Renaud RA, Friedrich TC, Gorski J. Estrogen causes cell death of estrogen receptor stably transfected cells via apoptosis. J Steroid Biochem Mol Biol. 1998;67:327-32.
11. Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17 beta-estradiol. J Natl Cancer Inst. 2001;93:1714-23.
12. Lewis JS, Meeke K, Osiop C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746-59.
13. Fan P, Griffith OL, Agboke FA, et al. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73:4510-20.
14. Umekita Y, Hiipakka RA, Kokontis JM, Liao S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci. 1996;93:11802-7.
15. Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941-53.
16. Chuu CP, Hiipakka RA, Fukuchi J, et al. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082-4.
17. Chuu CP, Kokontis JM, Hiipakka RA, et al. Androgen suppresses proliferation of castration-resistant LNCaP 104-R2 prostate cancer cells through androgen receptor, Skp2 and c-myc. Cancer Sci. 2011;102:2022-8.
18. Soto AM, Sonnenschein C. The two faces of Janus: sex steroids as mediators of both cell proliferation and cell death. J Natl Cancer Inst. 2001;93:1673-75.
19. Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028-36.
20. Liu H, Lee ES, Gajdos C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586-97.
21. Jordan VC. The 38th David A. Karnofsky Lecture: The paradoxical actions of estrogen in breast cancer – survival or death? J Clin Oncol. 2008;26:3073-82.
22. Jordan VC, Ford LG. Paradoxical clinical effect of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res. 2011:4:633-7.
23. Haddow A. David A. Karnofsky Memorial Lecture. Thoughts on chemical therapy. Cancer. 1970:26:737-54.
24. Yuan S, Trachtenberg J, Mills GB, et al. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304-11.
25. Shen R, Sumitomo M, Dai J, et al Androgen-induced growth inhibition of androgen receptor expressing androgen-independent prostate cancer cells is mediated by increased levels of neutral endopeptidase. Endocrinology. 2000;141:1699-1704.
26. Niu Y, Altuwaijri S, Lai K-P, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci. 2008;105:12182-7.
27. Cinar B, Koeneman KS, Edlund M, et al. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Res. 2001;61:7310-17.
28. Zhau HYE, Chang S-M, Chen B-Q, et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci. 1996;93:14152-5157.
29. Hara T, Nakamura K, Araki H, et al. Enhanced androgen receptor signaling correlates with the androgen-refractory growth in a newly established MDA PCa 2b-hr human prostate cancer cell line. Cancer Res. 2003;63:5622-8.
30. Thelen P, Heinrich E, Bremmer F, et al. Testosterone boosts for treatment of castration resistant prostate cancer: an experimental implementation of intermittent androgen deprivation. Prostate. 2013;73:1699-709.
31. Sun S, Sprenger CCT, Vessella RL, et al. Castration resistance is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715-30.
32. Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci. 2010;107:16759-65.
33. Litvinov IV, Vander Griend DJ, Antony L, et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci. 2006;103:15085-90.
34. Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72:1491-505.
35. Noble RL. Hormonal control of growth and progression in tumors of Nb rats and a theory of action. Cancer Res. 1977;37:82-94.
36. Foulds L. Neoplastic development. New York: Academic Press Inc. 1969;1:41-75.
37. Bruchovsky N, Rennie PS, Coldman AJ, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275-82.
38. Akakura K, Bruchovsky N, Goldenberg SL, et al. Effects of intermittent androgen suppression on androgen-dependent tumors; apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782-90.
39. Sato N, Gleave M, Bruchovsky N. Intermittent androgen suppression delays progression to androgen-independent regulation of prostate-specific antigen gene in the LNCaP prostate tumour model. J Steroid Biochem Mol Biol. 1996;58:139-46.
40. Feltquate D, Nordquist L, Eicher C, et al. Rapid androgen cycling as treatment for prostate cancer. Clin Cancer Res. 2006;12:7414-21.
41. Morris MJ, Huang D, Kelly WK, et al. Phase I trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. Eur Urol. 2009;56:237-44.
42. Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase I study of testosterone replacement for patients with low risk castrate resistant prostate cancer. Eur Urol. 2009;56:97-103.
43. Mathew P. Prolonged control of progressive castration-resistant metastatic prostate cancer with testosterone replacement therapy: the case for a prospective trial. Ann Oncol. 2008;19:395-403.
44. Sciarra A, Abrahamsson PA, Brausi M, et al. Intermittent androgen-deprivation therapy in prostate cancer: a critical review focused on phase 3 trials. Eur Urol. 2013;64:722-30.
45. Seftel AD. Male hypogonadism. Part I: Epidemiology of hypogonadism. Int J Impot Res. 2006;18:115-20.
46. Feneley MR, Carruthers M. Is testosterone treatment good for the prostate? Study of safety during long-term treatment. J Sex Med. 2012 9:2138-49.
47. Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451-7.
48. Morgantaler A, Lipshultz LI, Bennett R, et al. Testosterone therapy in men with untreated prostate cancer. J Urol. 2011;185:1256-61.
49. Prehn RT. On the prevention and therapy of prostate cancer by androgen administration. Cancer Res. 1999;59:4161-4.
50. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189:S26-S33.
51. Khera M, Crawford D, Morales A, et al. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65:115-23.
52. Sartor OA, Tangen CM, Hussain MHA, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer. Cancer. 2008;112:2393-400.
53. Hayward SW, Cunha SR. The prostate: development and physiology. Radiol Clin North Am. 2000;38:1-14.
54. Niu Y, Chang T-M, Yeh S, et al. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29:3593-604.
55. Qin J, Liu X, Laffin B, et al. The PSA -/lo prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell. 2012;10:1-14.
56. Bhasin S, Jasuja R. Selective androgen receptor modulators (SARMs) as function promoting therapies. Curr Opin Clin Nutr Metab Care. 2009;12:232-40.
57. Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;84:3459-62.
58. Green SM, Mostaghel EA, Nelson PS. Androgen action and metabolism in prostate cancer. Mol Cell Endocrinol. 2012;360:3-13.
59. Carter AC, Sedransk N, Kelley RM, et al. Diethylstilboestrol: recommended dosages for different categories of breast cancer patients. Report of the Cooperative Breast Cancer Group. JAMA. 1977;237:2079-85.