Brachytherapy or Surgery? A Composite View
The comparison of brachytherapy and surgery may be done on several levels. This review focuses the comparison on toxicity, the “soft” endpoints of biochemical relapse-free survival and clinical relapse-free survival, and the “hard” endpoint of prostate cancer–specific mortality.
ABSTRACT: The treatment of prostate cancer presents the patient and clinician with several options. The choice of modality is often the most difficult. For low- and intermediate-risk disease, the choice is often between surgery and brachytherapy because the other major option, external-beam radiotherapy, has toxicity that generally only makes sense in the risk/benefit analysis for high-risk prostate cancer. The comparison of brachytherapy and surgery may be done on several levels. This review will focus the comparison on toxicity, the “soft” endpoints of biochemical relapse-free survival and clinical relapse-free survival, and the “hard” endpoint of prostate cancer–specific mortality.
As treatments for prostate cancer, brachytherapy and surgery share logistics in that they both involve a procedure that may be performed in one intervention. Beyond that, one sees similarities and differences depending on the point of comparison being examined. The ideal dataset to use for such a comparison would be from a well-done randomized trial.
Unfortunately, no such trial exists. Some trials along these lines have randomized patients to either surgery or external-beam radiotherapy, but they yielded results that are not widely accepted because of small sample sizes, significant imbalances in pretreatment characteristics between groups, and randomization artifacts.[1,2] An attempt to compare brachytherapy and surgery in a randomized setting was closed due to poor accrual.[3] Also, none of these trials made sufficient plans to assess toxicity beyond simple investigator-reported grading systems. As a result, one must appeal to less robust data for analysis.
Despite the lack of randomized data, it is possible to sift through the literature for good quality research that will provide a basis for analysis. If one limits the inclusion of such research to prospective trials as well as retrospective trials with adequate follow-up and sufficient numbers of patients, a solid comparison of brachytherapy and surgery for prostate cancer may be made. This report will focus the comparison on toxicity, biochemical relapse-free survival (bRFS), clinical relapse-free survival (cRFS), and prostate cancer–specific mortality.
Toxicity
Most major cooperative groups have toxicity grading systems that tend to suffer from the same flaw: they are physician/investigator-reported. One recently completed prospective trial-the Prostate Cancer Outcomes and Satisfaction with Treatment Quality Assessment (PROST-QA)-used a validated, patient-reported questionnaire on toxicity. The 2-year data from this trial were first reported in 2008.[4] A careful review of its findings will disclose the early toxicity profiles of each of the major modalities.
The Prost-QA trial is an inception cohort study in which the patient and partner were asked to answer a validated questionnaire that measured the change from baseline of various quality-of-life domains. The five specific domains were: sexual, urinary incontinence, urinary irritation or obstruction, bowel or rectal, and vitality or hormonal. Patients with localized prostate cancer were offered their choice of therapy and the option to participate in the trial at one of six major cancer centers nationwide. The questionnaire was given at baseline and then at months 2, 6, 12, and 24 after therapy. While the trial included patients treated with external-beam radiotherapy as well as brachytherapy and surgery, we will focus on just the results for the brachytherapy and surgery patients.
The common finding in assessing the brachytherapy and surgery patients is the consistent nadir in quality-of-life score at the 2-month point for each of the affected domains. Generally, the quality-of-life score rebounded in subsequent months. In the sexual domain a major difference was seen in the quality-of-life score between brachytherapy and surgery patients. The surgery patients had a higher baseline sexual score (70–80), while the brachytherapy patients had a baseline of 60–70. At the 2-month assessment, the surgery patients’ sexual score fell to ~20, and at 2 years it was 20–40 (with the lower number being for those who did not receive a nerve-sparing operation). In contrast, the brachytherapy patients’ score fell to 45–50 at 2 months and was about the same at 2 years. While both modalities suffered a loss in this domain, the surgery patients took a greater loss relative to baseline. At 2 years, both modalities had essentially the same sexual score.
Another quality-of-life domain in which there was a significant difference between surgery and brachytherapy is that of urinary incontinence. Both modalities started with the same baseline of ~95. The expected drop in the domain score was seen in both groups; however, the surgery patients’ score fell the furthest (~50 for surgery vs ~82 for brachytherapy). Recovery occurred for both groups, but the surgery patients were at ~75 at 2 years, whereas at the same point, the brachytherapy patients were at ~90.
In contrast to the two previously discussed domains, brachytherapy fared worse in both urinary irritation or obstruction and bowel or rectal symptoms. Specifically, while both modalities started at the same baseline of ~90 for the urinary irritation or obstruction domain, the surgery patients were still at baseline at 2 years whereas the brachytherapy patients fell to ~80 by 2 years. In the bowel or rectal domain, both modalities again began at ~97, but the brachytherapy patients’ score fell to ~90 at 2 years and the surgery patients maintained their baseline function.
In summary, patients treated with each modality had an acute decrement in their quality of life, and there were unique, modality-dependent patterns to this effect in the long term. The surgery patients had a worse 2-year outcome relative to sexual function and urinary incontinence. The brachytherapy patients had a worse 2-year outcome when comparing urinary irritation or obstruction and bowel or rectal function.
Biochemical and Clinical Relapse-Free Survival
As stated earlier, no prospective randomized trials have addressed biochemical and clinical relapse-free survival in prostate cancer patients. Single-institution and multi-institutional retrospective reports of single-modality outcomes are available. At Cleveland Clinic, we maintain an inception cohort trial for all of our prostate cancer patients. We will use both of these sources to make the comparison between brachytherapy and surgery for the “soft” endpoints of bRFS and cRFS.
The first difficulty in assessing outcome relative to bRFS and cRFS is the dependence of the endpoints on the virulence of the cancer. Most investigators understand this, but it is still difficult to find reports that adhere to reproducible, accepted methods of stratifying patients according to virulence. We will try to review the literature with an emphasis on risk stratification. The second problem is defining bRFS and cRFS. Various reports use various measures of these endpoints. This is partly due to the era of reporting and partly due to institutional bias. We will do our best to compare similar groups of patients from the literature using similar endpoint assessments.
To bring this discussion to a close, we plan to use Cleveland Clinic data in a way that conforms to such standards for outcome reporting as exist in this field. Specifically, we will use the criteria put forward by the National Comprehensive Cancer Network (NCCN) for risk stratification and confine ourselves to the low- and intermediate-risk groups.[5] We will use a prostate-specific antigen (PSA) nadir posttherapy plus 2 ng/mL as the definition for biochemical failure following brachytherapy (Phoenix definition).[6] For the surgery patients, we will use a threshold of 0.4 ng/mL following therapy as the definition of biochemical failure.[7] The definition for clinical failure following both brachytherapy and surgery will be the same-biopsy-proven local or distant recurrence or image-defined distal recurrence.
A tabular summary of the bRFS results reported according to the previously discussed parameters is nearly impossible for the reasons mentioned above. One can perform a nontabular assessment, but attempts to compare patients according to risk groups or endpoint definitions are limited and inexact. Considering this, one of the best reports on brachytherapy results was published by Zelefsky et al.[8] In this multi-institutional report, they used the NCCN risk groupings and the Phoenix definition of bRFS. At 8 years, the bRFS was 74% for the low-risk population and 61% for the intermediate-risk group. The median follow-up was 63 months. These results are much worse than most individual institutional reports, where 90 % and 85% bRFS rates are routinely reported for low- and intermediate-risk groups, respectively. In the report by Zelefsky et al, however, only patients with a minimum follow-up of 60 months were included. Another often-quoted report is by Sylvester et al.[9] While this study had a long median follow-up of 113 months, it reported on brachytherapy combined with external-beam radiotherapy.
For surgery, the Johns Hopkins series is frequently referenced,[10] although it is only a single-institution report. Moreover, it reports patient outcomes relative to stage, initial PSA, postoperative Gleason score, and so forth, not by stratified NCCN risk groups, making comparisons difficult. The median follow-up for this series is 6.3 years. For patients with a PSA < 10 ng/mL, the 10-year bRFS was ~80%. For patients with T1c disease, the bRFS was ~76%. These findings in low-risk patients correlate with brachytherapy results quite well. It is not possible to tease intermediate-risk results from their report. A study from Washington University offers some comparison, but these investigators report on the combination of low- and intermediate-risk groups.[11] The median follow-up is 36 months. The bRFS for the dataset is ~80%. Again, this correlates well with brachytherapy results.
FIGURE 1
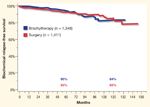
Biochemical Relapse-Free Survival in Low-Risk Prostate Cancer Patients FIGURE 2
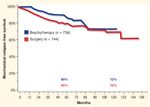
Biochemical Relapse-Free Survival in Intermediate-Risk Prostate Cancer Patients
None of these reports contain comparisons among modalities from a single institution with similar follow-up. The Cleveland Clinic’s inception cohort trial for prostate cancer patients has been maintained since the early 1990s. As a result, we are able to provide comparisons among contemporaneously treated patients with similar follow-up. Figures 1 and 2 depict Kaplan-Meier estimations of bRFS for the low- and intermediate-risk patients treated at Cleveland Clinic from 1996 to the present. Note that the outcome for both modalities is the same within the risk groups.
FIGURE 3
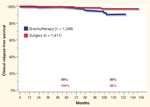
Clinical Relapse-Free Survival in Low-Risk Prostate Cancer Patients FIGURE 4
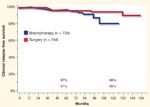
Clinical Relapse-Free Survival in Intermediate-Risk Prostate Cancer Patients
It is far more difficult to get a good grasp of cRFS from the literature. Han et al reported a metastasis-free survival rate of 82% for their combined series of mixed-risk groups.[10] Heranadez et al updated the study by Han et al with a select group of favorable patients, reporting a 99.5% cRFS for their combined series.[12] Few others assess this endpoint. The comparison of cRFS among modalities and within risk groups from Cleveland Clinic is shown in Figures 3 and 4. Once again, the outcome is nearly identical between brachytherapy and surgery. Despite the difficulty in assessing bRFS and cRFS, it seems appropriate to conclude that both brachytherapy and surgery offer similar very good outcomes in terms of these “soft” but often-quoted endpoints.
Prostate Cancer–Specific Mortality
FIGURE 5
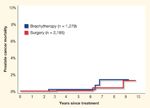
Prostate Cancer-Specific Mortality by Treatment in Low- and Intermediate-Risk Patients
The assessment of prostate cancer–specific mortality is rarely done, yet it is the least ambiguous endpoint one can assess. For the combined-risk group in their surgical series, the Hopkins team reported a prostate cancer–specific mortality of 10% at 15 years.[10] In a study by D’Amico et al, the prostate cancer–specific mortality was ~2%–3% for intermediate-risk patients treated with surgery at 7 years.[13]
A cumulative incidence plot of the Cleveland Clinic low- and intermediate-risk patients is depicted in Figure 5. The Cleveland Clinic data agree with D’Amico et al, and both are significantly better than the Hopkins series. This is very likely a function of the era in which the patients were treated and the length of follow-up. The Hopkins patients had more opportunity to experience treatment failure and were treated before screening culled out the more advanced patients. As a result, the relatively poor results are not unexpected. In general, it can be said that the prostate cancer–specific mortality for surgery and brachytherapy is the same.
Conclusion
When comparing surgery and brachytherapy for the treatment of low- and intermediate-risk prostate cancer, the differences are minimal. The primary differences are in the toxicity profiles of the treatments. In particular, surgery is associated with more incontinence and sexual dysfunction than brachytherapy, while brachytherapy carries a greater risk of urinary irritation and obstruction and rectal problems. The bRFS, cRFS, and prostate cancer–specific mortality are nearly identical.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Editor’s Note:This article is part of a special series, Controversies in the Management of Localized Prostate Cancer, which began in the September issue of ONCOLOGY. Guest edited by Dr. Derek Raghavan, each article in the series takes a particular position on optimal patterns of care for early-stage prostate cancer.
References:
1. Akakura K, Isaka S, Akimoto S, et al: Long-term results of a randomized trial for the treatment of stages B2 and C prostate cancer: Radical prostatectomy versus external beam radiation therapy with a common endocrine therapy in both modalities. Urology 54:313-318, 1999.
2. Paulson DF, Lin GH, Hinshaw W, et al: Radical surgery versus radiotherapy for adenocarcinoma of the prostate. J Urol 128:502-504, 1982.
3. Klein EA, Ciezki J, Kupelian PA, et al: Outcomes for intermediate risk risk prostate cancer: Are there advantages for surgery, external radiation, or brachytherapy? Urol Oncol 27:67-71, 2009.
4. Sanda MG, Dunn RL, Michalski J, et al: Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358:1250-1261, 2008.
5. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology-prostate cancer, v.2.2009. Available at www.nccn.org. Accessed August 24, 2009.
6. Roach M, Hanks G, Thames H, et al: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys 65:965-974, 2006.
7. Stephenson AJ, Kattan MW, Eastham JA, et al: Defining biochemical recurence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J Clin Oncol 24:3973-3978, 2006.
8. Zelefsky MJ, Kuban DA, Levy LB, et al: Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 67:327-333, 2007.
9. Sylvester JE, Grimm PD, Blasko JC, et al: 15-year biochemical relpase free survival in clinical stage T1-T3 prostate cancer following combined external beam radiotherapy and brachytherapy; Seattle experience. Int J Radiat Oncol Biol Phys 67:57-64, 2007.
10. Han M, Partin AW, Pound CR, et al: Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy The 15-year Johns Hopkins experience. Urol Clin North Am 28:555-565, 2001.
11. Antenor JAV, Roehl KA, Eggener SE, et al: Preoperative PSA and progression-free survival after radical prostatectomy for stage T1c disease. Urology 66:156-160, 2005.
12. Hernandez DJ, Nielsen ME, Han M, et al: Natural history of pathologically organ-confined (pT2), Gleason score 6 or less, prostate cancer after radical prostatectomy. Urology 72:172-176, 2008.
13. D’Amico AV, Chen M-H, Catalona WJ, et al: Prostate cancer-specific mortality after radical prostatectomy or external beam radiation therapy in men with 1 or more high-risk factors. Cancer 110:56-61, 2007.