Capecitabine Improved Safety Profile Maintained in Older Pts With Colon Ca
This special “annual highlights” supplement to Oncology News International is acompilation of some of the major advances in the management of gastrointestinalcancers during 2003–2004, as reported in ONI. Guest editor Dr. James L. Abbruzzesecomments on the reports included herein and discusses advances in the clinicalmanagement of GI cancers, with a focus on developments in targeted therapy, newcombinations, adjuvant therapy, and what to watch for in 2004.
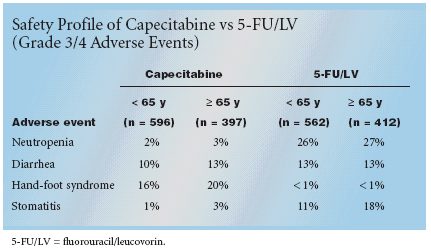
COPENHAGEN-Results ofthe X-ACT trial of oral capecitabine(Xeloda) vs fluorouracil/leucovorin(5-FU/LV) as first-line adjuvant therapyfor metastatic colon cancer showedthat capecitabine had an improvedsafety profile, compared with 5-FU/LV, overall and in patients age 65 andolder. Christopher Twelves, MD, pro-fessor of clinical cancer pharmacology,University of Leeds and Tom ConnorsCancer Research Centre, Bradford,UK, reported the results at ECCO12, the European Cancer Conference.The data have also been published inAnnals of Oncology (14:1735-1743,2003).The study randomized patientswith resected Dukes' C colon cancerto receive 24 weeks of treatment witheither capecitabine 1,250 mg/m2 twicedaily on days 1 to 14 every 21 days (n= 993) or IV bolus 5-FU 425 mg/m2plus IV leucovorin 20 mg/m2 on days1 to 5, repeated every 28 days (n =974). Efficacy results are expected tobe available sometime this year.In a planned safety analysis, patientsreceiving capecitabine had significantlyless grade 3/4 neutropenia,febrile neutropenia/sepsis, and stomatitis(P < .001) but more grade 3hand-foot syndrome than those treatedwith 5-FU/LV. Capecitabine had asimilar, favorable safety profile in patientsless than 65 years of age and inthose 65 or older (see Table).
In their Annals of Oncology report,the investigators stressed that "goodtolerability is a particularly importantconsideration when chemotherapy isadministered in the adjuvant setting,"especially considering recent resultsfrom trials evaluating 5-FU/LV in combinationwith irinotecan (Camptosar)or oxaliplatin (Eloxatin).For example, in the MOSAIC trialcomparing bolus/infusional 5-FU/LVand oxaliplatin (FOLFOX4) vs infusional5-FU/LV (the de Gramont regimen)as adjuvant therapy for patientswith stage II-III colon cancer, FOLFOX4produced a significant improvementin 3-year disease-free survivalbut, the investigators said, "at the expenseof a higher incidence of grade 3or 4 neutropenia, and 29% of patientsexperienced long-term (> 1 year) lowgradeneurotoxicity."The X-ACT researchers concludedthat in metastatic colon cancer"capecitabine is an attractive agent toreplace 5-FU/LV as the backbone ofadjuvant combination treatment forfurther studies."