Challenges of Treating a Patient With Advanced Prostate Cancer During the COVID-19 Pandemic
Carril-Ajuria Department of Medical Oncology, Hospital Universitario 12 de Octubre, Madrid, Spain.

Remolina-Bonilla Department of Hematology and Oncology, Instituto Nacional de Ciencias Medicas y Nutrición Salvador Zubirán, Mexico City, Mexico.

M. Bourlon Department of Hematology and Oncology, Instituto Nacional de Ciencias Medicas y Nutrición Salvador Zubirán, Mexico City, Mexico.

C. Bourlon Department of Hematology and Oncology, Instituto Nacional de Ciencias Medicas y Nutrición Salvador Zubirán, Mexico City, Mexico.

Velasco Department of Medical Oncology, Hospital Universitario 12 de Octubre, Madrid, Spain.

Carretero-González Department of Medical Oncology, Hospital Universitario 12 de Octubre, Madrid, Spain.

THE CASE
A 78-year-old man had a medical history of hypertension, atrial fibrillation, chronic kidney disease, and metastatic castration-resistant prostate cancer (CRPC). He had progressed to first-line therapy for CRPC with abiraterone plus androgen-deprivation therapy (ADT) and as second-line therapy he was being treated with docetaxel, with biochemical progression in his last prostate-specific antigen measurement.
He was admitted to the hospital on April 2020, in the middle of the coronavirus disease 2019 (COVID-19) pandemic, because of painful bone lesions and deterioration of renal function. His fourth chemotherapy cycle and monthly zoledronic acid had been administered 4 weeks prior to admission.
Four days into this hospitalization, the patient developed respiratory symptoms characterized by shortness of breath and cough. Pulse oximeter confirmed by blood gas analysis revealed hypoxemia, requiring supplemental oxygen via nasal cannula at 2 L/min to maintain an oxygen saturation level of more than 90%. A chest x-ray compared with the prior one taken a month ago showed bilateral patchy opacities predominantly on the right lung (Figures 1A and B). Nasopharyngeal swab specimens were positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR).
Figure 1. Chest x-ray comparison of normal study 1 month prior to admission, (A) at time of respiratory symptoms initiation showing bilateral patchy infiltrates with right lung predominant distribution, (B) and 48 hours after treatment initiation at time of symptoms aggravation consistent with progression of bilateral lung infiltrates (C).
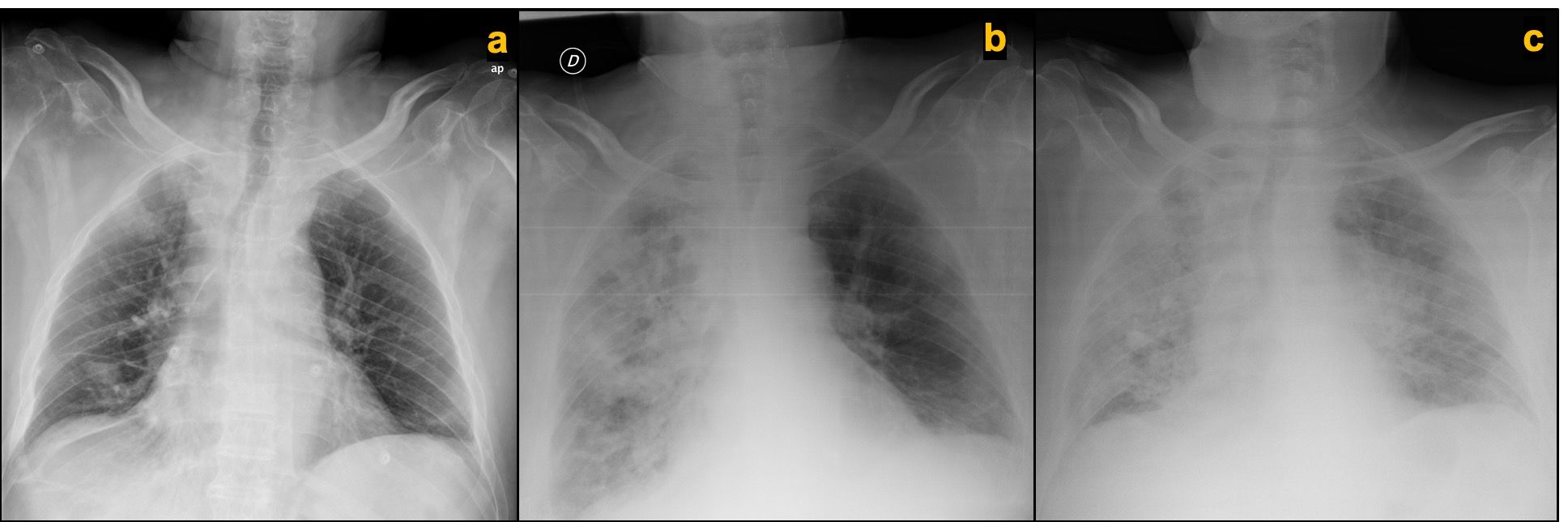
Additional laboratory tests revealed leukocytosis (14.900 cells/µl), lymphopenia (600 cells µl), elevated C-reactive protein (31 mg/dL), elevated lactate dehydrogenase (580 U/L), and worsening of kidney function: creatinine 2.95 mg/dL (baseline creatinine, 1.70 mg/dL),consistent with diagnosis of severe bilateral COVID-19 pneumonia with acute respiratory failure.
Treatment with antibiotics with aztreonam, ciprofloxacin, and linezolid (because of his β-lactam allergy); antiretroviral therapy with lopinavir-ritonavir; and low-molecular-weight heparin (LMWH) thromboprophylaxis was started. After 48 hours of full medical therapy, his clinical condition deteriorated and his oxygen requirements increased. A follow-up chest x-ray revealed worsening bilateral patchy infiltrates (Figure 1C). A multidisciplinary discussion among the internal medicine, pulmonology, intensive care, and medical oncology teams was held. The patient was not considered a candidate for mechanical ventilation due to a shortage of intensive care unit beds as a result of the pandemic, neoplastic disease in progression with second-line therapy, poor performance status, and coexisting comorbidities. He was sent home with palliative care with quarantine precautions for himself and his family, and died 3 days later due to respiratory failure.
Which of the following statements are true about cancer and COVID-19?
A. Patients with cancer have an increased risk of infection and related complications.
B. Transmembrane protease serine 2 (TMPRSS2) inhibition may be a protective factor for infection in patients with prostate cancer.
C. Predictive factors for mortality in patients with cancer are similar to those in all patients with COVID-19.
D. Palliative care delivery has changed with this pandemic.
E. All of the above are true.
CORRECT ANSWER: E. All of the above are true.
Discussion
COVID-19 is a viral respiratory illness caused by SARS-CoV-2 and was declared a global pandemic on March 11, 2020. It has affected all medical disciplines. Treating patients effectively within this health crisis has now become a major challenge for clinicians. Cancer care delivery is no exception, as all aspects of its practice––from screening to palliative care––have had to be modified, with inevitable consequences for medium- and long-term prognosis.1,2 Worldwide, the practice of oncology has now changed.3 Oncologists face challenges such as delays in surgical procedures, preferential use of oral agents instead of parenteral therapy regimens, and ensuring that telemedicine visits are available. Meanwhile, patients are experiencing travel restrictions on account of quarantine policies, and are fearful of visiting hospitals because of the exposure risk and unavailability of outpatient services, among other concerns.4
Present data suggest that cancer patients possess an increased risk for severe COVID-19 and its complications, including death.5-8 Initial information from China revealed a higher infection rate among cancer patients compared with all COVID-19 cases (0.79% vs 0.37%), with an odds ratio (OR) of 2.31 (95% CI, 1.89-3.02)8, and other studies describe higher infection rates approximately 6 times greater in patients with malignancy compared with the general population.9,10
In Chinese studies, 13.4% to 41.3% of COVID-19 cases in patients with cancer were believed to occur through hospital-associated transmission. The most common neoplasm associated with this infection was lung cancer (22.4%-28% of cases). One- to two-thirds of patients (39.2%-64.2%) had coexisting chronic diseases. Almost all patients (> 94%) had abnormal findings on computed tomography (CT) at COVID-19 disease presentation, and the proportion of patients who had received oncologic treatment within the last month was 21.4% to 34.3%. Acute respiratory distress syndrome developed in 15.6% to 28.6% of patients with cancer, with mechanical ventilation requirement and intensive care unit (ICU) admission rates of 35.7% to 41.8% and 21.4%, respectively. Compared with the overall population, patients with cancer developed more severe illness (47.8%-53.6% vs 19%), had a more rapid deterioration (13 vs 43 days), and had a tenfold higher case-fatality rate (25%-28.6% vs 2.3%).5-8,11 Patients receiving any modality of oncologic treatment within 14 days of COVID-19 diagnosis demonstrated an increased risk for admission to an ICU, mechanical ventilation requirement, or death (HR, 4.1; 95% CI, 1.1-15.3; P = .037), whereas patients with a patchy consolidation on CT at presentation showed an increased risk of complications or death (HR, 5.4; 95% CI, 1.5-19.7; P = .010).6
Currently, the United States has the largest number of COVID-19 cases of all countries, with New York City being the country’s first epicenter. The city’s data incorporated new predictive factors for adverse outcomes in patients with cancer who also have COVID-19. The highest case-fatality rate occurred in those with lung cancer (55%). Genitourinary and breast cancers were associated with lower mortality (14% and 15%, respectively). Factors related with increased mortality included the following: age (76 vs 66 years, P = .0006); comorbidities, such as hypertension (P = .047); coronary artery disease (P = .012);chronic heart failure (P = .019); chronic lung disease (P < .001); and metastatic disease (P = .06). Laboratory findings such as anemia (P = .047), higher absolute neutrophil counts (P = .017), elevated D-dimer levels (P = .002), lactic acid (P = .001), and lactate dehydrogenase (LDH; P = .01) were significantly associated with worse survival. In the multivariate analysis of factors associated with case fatality, being aged 65 or younger was a protective factor for death (OR, 0.23; 95% CI, 0.07-0.61; P = .005), and factors such as ICU admission (OR, 4.83), D-dimer (OR, 1.15), lactic acid (OR, 1.99), LDH (OR, 1003), and comorbidity score (OR, 1.52) were all related to a poor prognosis with statistical significance. New York City data also reaffirm that cancer patients have a 2.45-fold elevated fatality rate (P < .001) compared with the general population.12
Therefore, statements A and C are correct options.
Regarding prostate cancer, current information is based on Italy’s experience, which revealed that all male patients with cancer had an increased risk of SARS-CoV-2 infection (OR, 1.79; 95% CI, 1.62-1.98; P < .0001) with higher hospitalizations and mortality rates. When the prostate cancer population (n = 118) was divided according to treatment with ADT versus no ADT, a significantly higher risk of COVID-19 was identified in the non-ADT–treated group (OR, 4.05; 95% CI, 1.55-10.59; P = .006). Additionally, when other types of cancer were compared with the ADT group, an increased risk was sustained (OR, 5.17; 95% CI, 2.02-13.40; P = .001).13 This may be explained through the expression of TMPRSS2––mainly in the prostate, but also in the lung––which is upregulated by androgen receptor,
facilitates viral entry and replication, and cleaves angiotensin converting enzyme 2 for augmented viral entry.14,15 Androgen sensitivity could be a determinant factor in COVID-19 severity and ADT downregulates this phenomenon.16 Thus, option B is also a valid statement.
The data in relation to COVID-19 and cancer must be interpreted with caution because of the retrospective nature and heterogeneity of the studies. In addition, these reports do not include information regarding do-not-resuscitate or do-not-intubate orders, which could impact mortality and severe events calculation.
COVID-19 has exposed the weaknesses of health care systems in several areas including palliative care, which has faced many challenges during this pandemic.17 One example is Italy, where the requests of home care assistance doubled in a system that was already saturated. There also was a lack of the equipment required to protect patients and health care workers, limited patient follow-up at home, and, in some cases, a cessation of activities by hospices.18 In Singapore, palliative care teams were disassembled so that physicians could work on the front lines.19 Furthermore, prohibiting the presence of a family member resulted in many patients dying alone, which has profoundly affected the palliative care carried out by specialists, in which a multidisciplinary approach and family involvement is essential.
For patients with cancer, the management of COVID-19 needs to be adjusted according to the type of cancer, disease stage, expected survival, line of treatment, and previous performance status.20 These have always been important when patients with cancer face an acute illness; however, they become more crucial when the patient and the system are unable to adapt, and resources are limited, as has been the case during this pandemic. Currently, clinicians must discuss care goals in advance with their patients who have cancer and allow them to make their own decisions.17,21
Therefore, option D is also correct.
Would the treatment the 78-year-old patient received have been different had his last progression not occurred during the pandemic? As clinicians, we cannot avoid thinking about this question. Perhaps the outcome would have been different. For instance, the access to an ICU would not have been so restricted, his condition could have improved, and he could have reached a better performance status that might have allowed for further therapeutic interventions. Nowadays, there are many treatment options for metastatic CRPC; hence, the patient would have had an alternative for a third-line treatment such as enzalutamide or cabazitaxel.
In conclusion, our clinical case is a good example of the combination of several predictive risk factors such as age, coexisting comorbid conditions, laboratory findings, and recent oncological treatment (chemotherapy) that lead to worse outcomes in patients with cancer who have COVID-19.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Corresponding author:
Maria T Bourlon MD, MHS
Vasco de Quiroga No. 15., Belisario Dominguez Sección XVI, Tlalpan, C.P. 14080, Ciudad de México, México
maitebourlon@gmail.com
About the SERIES EDITORS:
Maria T. Bourlon, MD is Associate Professor, Head Urologic Oncology Clinic, National Researcher. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Mexico City, Mexico. She is also a Member of ASCO’s IDEA Working Group.
E. David Crawford, MD, is Chairman, Prostate Conditions Education Council; Editor in Chief, Grand Rounds in Urology; and Professor of Urology, University of California San Diego, La Jolla, California.
References
1.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750-751. doi:10.1016/S1470-2045(20)30265-5
2.The Lancet Oncology. Safeguarding cancer care in a post-COVID-19 world. Lancet Oncol. 2020;21(5):603. doi:10.1016/S1470-2045(20)30243-6
3.Waisberg F, Enrico D, Angel M, Chacón M. Cancer treatment adaptations in the COVID-19 era. JCO Oncol Pract. 2020 Jun;16(6):305-307. doi:10.1200/op.20.00218
4.Gao Z, Yang Y, Ding C, et al. Oncologist's perspective: when cancer encounters COVID-19. Oncologist. Published online May 15, 2020. doi:10.1634/theoncologist.2020-0296
5.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. doi:10.1016/S1470-2045(20)30096-6
6.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894-901. doi:10.1016/j.annonc.2020.03.296
7.Zhang HY, Wang LW, Chen YY, et al. A multicentre study of 2019 novel coronavirus disease outcomes of cancer patients in Wuhan, China. medRxiv. Published online April 15, 2020. doi:10.1101/2020.03.21.20037127
8.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108-1110. doi:10.1001/jamaoncol.2020.0980
9.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.04.006
10.Desai A, Sachdeva S, Parekh T, Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557-559. doi:10.1200/GO.20.00097
11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
12.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935-941. doi:10.1158/2159-8290.CD-20-0516
13.Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040-1045. doi:10.1016/j.annonc.2020.04.479
14.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. doi:10.1016/j.cell.2020.02.052
15.Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83(1):308-309. doi:10.1016/j.jaad.2020.04.032
16.Wambier CG, Goren A, Vaño-Galván S, et al. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev Res. Published online May 15, 2020. doi:10.1002/ddr.21688. doi:10.1002/ddr.21688
17.Singhai P, Rao K, Rao S, Salins NS. Palliative care for advanced cancer patients in the COVID-19 pandemic: challenges and adaptations. Cancer Research, Statistics, and Treatment. 2020;3(5):127-32. doi: 10.4103/CRST.CRST_130_20
18.Mercadante S, Adile C, Ferrera P, Giuliana F, Terruso L, Piccione T. Palliative care in the time of COVID-19. J Pain Symptom Manage. 2020;60(2):e79-e80. doi:10.1016/j.jpainsymman.2020.04.025
19.Koh MYH. Palliative care in the time of COVID-19: reflections from the frontline. J Pain Symptom Manage. 2020;60(1):e3-e4. doi:10.1016/j.jpainsymman.2020.03.023
20.Spicer J, Chamberlain C, Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020;17(6):329-331. doi:10.1038/s41571-020-0370-6
21.Curtis JR, Kross EK, Stapleton RD. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19). JAMA. 2020;323(18):1771-1772. doi:10.1001/jama.2020.4894
