Chemoradiation in NSCLC: Focus on the Role of Gemcitabine
Research to identify the optimal drugs for use in chemoradiotherapyhas led to the development of the potent radiosensitizing agentgemcitabine (Gemzar), which has exhibited excellent activity in non-small-cell cancer. When used in sequential chemoradiotherapy regimens,gemcitabine has been associated with response rates of 57% to68%. A full dose of gemcitabine (1,000 mg/m2) can be safely used asinduction therapy, and there is no definitive indication of enhancementof radiotoxicity. In addition, results from phase I/II trials supportthe efficacy of concurrent gemcitabine/radiation therapy in improvingoverall response rates and overall survival. Rates of 68%, 37%, and28%, respectively, for 1-, 2-, and 3-year survival have been reported forgemcitabine/cisplatin chemotherapy administered concurrently withradiotherapy. Although the optimal dose has yet to be determined, aweekly dose of 300 mg/m2 appears to be effective with an acceptabletoxicity level. Additional clinical trials are warranted to assess the longtermefficacy and safety of gemcitabine in combination with other chemotherapeuticagents and radiation therapy for treatment of non-smallcelllung cancer.
ABSTRACT: Research to identify the optimal drugs for use in chemoradiotherapyhas led to the development of the potent radiosensitizing agentgemcitabine (Gemzar), which has exhibited excellent activity in non-small-cell cancer. When used in sequential chemoradiotherapy regimens,gemcitabine has been associated with response rates of 57% to68%. A full dose of gemcitabine (1,000 mg/m2) can be safely used asinduction therapy, and there is no definitive indication of enhancementof radiotoxicity. In addition, results from phase I/II trials supportthe efficacy of concurrent gemcitabine/radiation therapy in improvingoverall response rates and overall survival. Rates of 68%, 37%, and28%, respectively, for 1-, 2-, and 3-year survival have been reported forgemcitabine/cisplatin chemotherapy administered concurrently withradiotherapy. Although the optimal dose has yet to be determined, aweekly dose of 300 mg/m2 appears to be effective with an acceptabletoxicity level. Additional clinical trials are warranted to assess the longtermefficacy and safety of gemcitabine in combination with other chemotherapeuticagents and radiation therapy for treatment of non-smallcelllung cancer.
The discovery that chemotherapyand radiation therapy separatelyimprove response ratesand survival in patients with non-small-cell lung cancer (NSCLC) hasspurred considerable interest in determiningthe optimal use of these treatmentmodalities. Initial researchdemonstrated that sequential chemoradiationtherapy consistently improvessurvival in locally advancedNSCLC compared with either radiationtherapy or chemotherapy alone.Chemoradiation:Sequential vs ConcurrentIn a series of clinical trials, the2-year overall survival ranged between19% to 25% in those receivingsequential chemoradiation therapycompared with 13% to 17% in patientstreated with radiotherapyalone.[1-4] Similarly, phase II studiesconducted in Japan showed a 2-yearsurvival rate of 36% for patients receivingchemoradiation therapy comparedwith 9% for chemotherapyalone; by 3 years, the respective survivalrates were 30% and 3%.[5] Thereis no role for chemotherapy alone instage III disease.Subsequently, six clinical trialsconducted in Europe, Asia, and theUnited States addressed the key issueof determining the optimal sequenceof chemotherapy and radiation therapy.[6-10] Regardless of what drug wasgiven or where the study was done,results consistently demonstrated thatconcurrent chemoradiotherapy providedlonger mean survival than sequentialchemoradiotherapy. Theaverage median survival was about14 months for sequential chemoradiotherapyand approximately 17months for concurrent radiochemotherapy(P < .05) (Figure 1; unpublisheddata). The survival benefitshave been maintained for as long as5 years.In one long-term study, a chemotherapyregimen of cisplatin (80 mg/m2on days 1 and 29), vindesine (3 mg/m2on days 1, 8, 29, and 36), and mitomycin(8 mg/m2 on days 1 and 29)was administered either before or atinstitution of radiotherapy (28 Gy) in323 patients with NSCLC. The 5-yearoverall survival rate was 9% for patientsreceiving sequential chemoradiationtherapy vs 19% for concurrentchemoradiation therapy.[6]
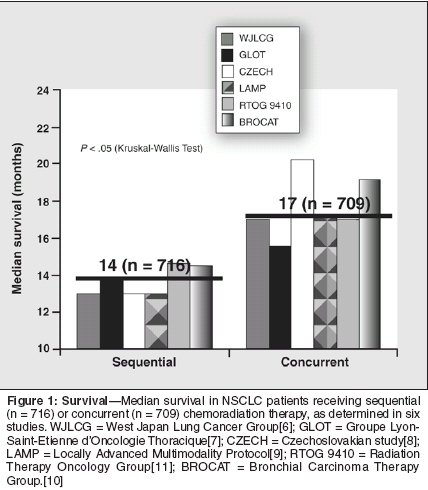
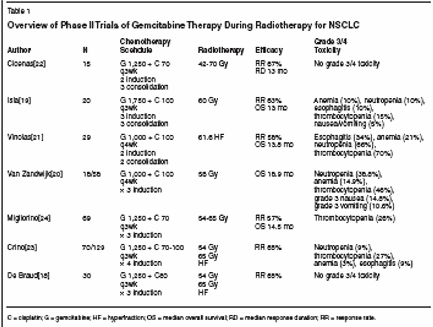
Similarly, in a study involving 610patients with NSCLC who were treatedwith either cisplatin (100 mg/m2on days 1 and 29) plus vinblastine (5mg/m2 on days 1, 8, 29, and 36) orcisplatin (50 mg/m2 on days 1, 8, 29,and 36) plus oral etoposide (50 mgbid for 10 weeks) with radiation (60to 69.6 Gy), the 5-year survival rateswere 12% and 21% for sequential andconcurrent chemoradiation therapy,respectively.[11] These findingsstrongly suggest that synergy betweentreatment modalities improves locoregionalcontrol and survival. The disadvantageis that enhanced toxicitymay restrict the ability to delivery fulldoses of both modalities. For that reason,concurrent chemoradiotherapymay be indicated for patients whoseperformance status is 0 or 1.Gemcitabine andRadiation TherapyResearch to identify the optimaldrugs for use in concurrent chemoradiotherapyhas lead to the developmentof a number of new agents, suchas paclitaxel, irinotecan (Camptosar),docetaxel (Taxotere), and vinorelbine(Navelbine). Among the more promisingnew options is the novel deoxycitidineanalog gemcitabine (Gemzar),which has demonstrated excellent single-agent activity in NSCLC.[12]In preclinical studies, gemcitabinehas exhibited cell phase specificity,primarily by killing the radioresistantcells undergoing DNA synthesis(S-phase cells) and also by blockingthe progression of cells through theG1/S-phase boundary.[13-17] The cytotoxiceffects of gemcitabine are attributedto the active diphosphate andtriphosphate nucleosides. Diphosphategemcitabine facilitates incorporationof triphosphate gemcitabine intoDNA, which ultimately inhibits DNAsynthesis and induces apoptosis.These cytotoxic effects rendergemcitabine a potent radiosensitizerfor NSCLC.[12] The sensitizer enhancementratio is ≥ 1.5.[13,14] Durationof sensitization after exposureexceeds 76 hours.[15,16] Sensitizationoccurs at subcytotoxic doses andis induced more rapidly with higherdoses.Gemcitabine inSequential TherapyGemcitabine is indicated in combinationwith cisplatin as first-linetherapy for locally advanced (stageIIIA or IIIB) or metastatic (stage IV)NSCLC.[17] When administeredin dosages between 1,000 and1,750 mg/m2, gemcitabine has beenassociated with response rates rangingfrom 57% to 68% (Table 1).[18-24] A major advantage of gemcitabineis its low toxicity when administeredsequentially as monotherapy or combinedwith cisplatin, followed by radiation.(It may be administered inpatients with low performance statusof 1 or 2.) However, gemcitabineshould be used with caution after radiotherapy,because it has been associatedwith radiation recall syndrome,a rare but serious phenomenon.[25]A full dose of gemcitabine (1,000mg/m2) can be used safely with cisplatinas induction therapy followedby radiation. In a potentially curativesetting, an interval of 1 to 4 weeks isrecommended between gemcitabineand radiotherapy to minimize the riskof radiation recall syndrome. In a palliativesetting, however, when radiotherapyis used to treat bonemetastases, the interval between chemotherapyand radiation therapy canbe reduced if necessary.
Gemcitabine and ConcurrentRadiation TherapyPhase I/II Trials
The activity of concurrent gemcitabineand radiation therapy in NSCLCwas initially demonstrated in a multiinstitutionalphase II pilot study conductedfrom October 1994 throughAugust 1995.[26] The treatmentregimen included gemcitabine at1,000 mg/m2/wk for 6 weeks, giventogether with radiation administeredas a 2-Gy fraction 5 days/wk up to amaximum dose of 60 Gy.[26] Sevenof the eight patients had a > 50%reduction in the primary tumor, andfour of five patients experienced aresponse in the nodes.The excellent activity of this regimen,however, was accompanied byserious toxicity. There were threetreatment-related deaths, two due topulmonary toxicity and one resultingfrom hemorrhage due to radiation necrosis.Three patients experiencedpneumonitis or severe esophagitis, andanother two patients experienced seriousradiation-induced side effects.Two factors were believed to be primarilyresponsible for this toxicity. Firstwas the large starting dose of gemcitabine,which had been chosen beforeresults of phase I dose-escalation studieshad been completed. Second wasthe large radiation treatment volume,which encompassed approximately5,000 cm3 for the initial volume and2,000 cm3 for the booster field. Thosevolumes are approximately twice thevolumes that are recommended in theUnited States (personal communication,A. Turrisi A, H. Choy, 2003).Subsequent phase I studies havedemonstrated that gemcitabine, indoses ranging from 100 to 600mg/m2/wk, are well tolerated duringradiation therapy.[27-30] The risk ofserious toxicity increased substantiallywith the dose, and the maximumtolerated dose ranged from 190 to350 mg/m2/wk.A recent randomized phase II studysupports administration of gemcitabinewith cisplatin during radiationtherapy for NSCLC.[31] Chemotherapy,consisting of cisplatin combinedwith gemcitabine, paclitaxel, or vinorelbine,was administered in twocycles of induction chemotherapy followedby two additional cycles of thesame drugs with concomitant radiotherapy.All 175 patients received fourcycles of cisplatin at 80 mg/m2 ondays 1, 22, 43, and 65. Dosage schedulesfor the three different treatmentarms were as follows: gemcitabine at1,250 mg/m2 on days 1, 8, 22, and 29,and 600 mg/m2 on days 43, 50, 64,and 71; paclitaxel at 225 mg/m2 for 3hours on days 1 and 22, and 135 mg/m2 on days 43 and 64; and vinorelbineat 25 mg/m2 on days 1, 8, 15, 22,and 29, and 15 mg/m2 on days 43, 50,64, and 71. Radiotherapy was institutedon day 43 at 2 Gy/d (total dose,66 Gy).
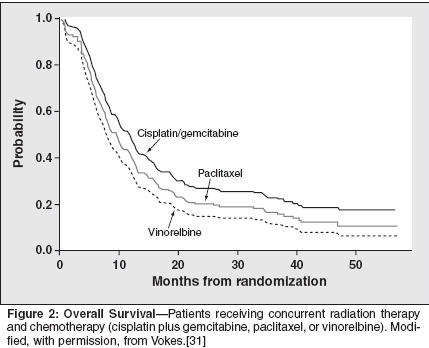
Response rates after completion ofradiotherapy were 74%, 67%, and73%, respectively, for the gemcitabine,paclitaxel, and vinorelbine arms.Median survival for all patients was17 months. Median progression-freesurvival was 8.4 months with gemcitabine/cisplatin, 9.1 months with paclitaxel/cisplatin, and 11.5 monthswith vinorelbine/cisplatin. Rates were68%, 37%, and 28% for 1-, 2-, and 3-year survival, respectively, for thegemcitabine arm; 62%, 29%, and 19%for the paclitaxel arm; and 65%, 40%,and 23% for the vinorelbine arm (Figure2). The study was too small toshow statistical significance in survivalrates between treatment groups,although the numerically higher3-year survival rate for the gemcitabine/cisplatin arm appears to be encouraging.Toxicities during induction chemotherapyconsisted primarily of grade3 or 4 granulocytopenia. Grade 3 or 4toxicities during concomitant chemoradiotherapymost frequently involvedthrombocytopenia, granulocytopenia,and esophagitis. Of the group receivingthe vinorelbine/cisplatin combination,10 patients experienced grade4 lung toxicity, and 1 patient died as aresult of treatment-related respiratoryfailure.Dose-Escalation Trial
The optimal dosage of gemcitabineduring radiation therapy forNSCLC is being assessed in an ongoing"Ping-Pong" trial (RTOG 0017),in which patients are recruited intotreatment sequences involving escalatingdosages of either gemcitabineplus carboplatin or gemcitabine pluspaclitaxel (unpublished data). However,as of publication, sequence B-the "Pong" portion of the trial-hasclosed due to safety concerns abouttoxicities.The original design was as follows.In sequence A, there are five dosagesof gemcitabine used in the sixgemcitabine/carboplatin treatmentarms: 300 mg/m2/wk (including onemonotherapy and one combinationtherapy arm), 450, 600, 750, and900 mg/m2/wk. Carboplatin will begiven at an area under the concentration-time curve (AUC) of 2 in fivetreatment arms, but not used in thesixth. Sequence B was designed toinclude six treatment arms involvinggemcitabine and paclitaxel, respectively,in dosages as follows: 300/30,450/30, 450/40, 600/40, 600/50, and750/50 mg/m2/wk. All arms would befollowed by two cycles of consolidatedchemotherapy, gemcitabine at1,000 mg/m2 weekly 2 out of 3, andcarboplatin at AUC 5.5.Patients will be recruited first intothe lowest-dose arm of sequence A(300 mg/m2/wk gemcitabine monotherapy).After recruitment is completedin the first arm, toxicity willbe assessed, while patients are beingentered into the lowest dose armof sequence B (gemcitabine at300 mg/m2/wk plus paclitaxel30 mg/m2/wk). While the toxicity ofthat regimen is being evaluated, patientsare recruited into the secondlowest dose arm in sequence A (gemcitabineat 300 mg/m2/wk pluscarboplatin AUC 2). Recruitmentwill continue at escalating dosage levelsuntil toxicity becomes unacceptable.Dose escalation continues as longas ≤ two of six patients experiencedose-limiting toxicity. The estimatedaccrual is 36 patients, six per treatmentarm.Preliminary toxicity data have beenderived from the first 24 patients, seveneach in the first three arms. Grade3/4 hematologic toxicity occurred infour of seven patients in arm 1 (gemcitabineat 300 mg/m2/wk monotherapy),five of seven patients in arm 2(gemcitabine at 300 mg/m2/wk pluspaclitaxel at 30 mg/m2/wk), and fourof seven patients in arm 3 (gemcitabineat 300 mg/m2/wk plus carboplatinat AUC 2). Pulmonary toxicity wasnoted only in two patients in arm 2.Since three patients in arm 2 had doselimitingtoxicities, researchers concludedthat the combination ofgemcitabine plus paclitaxel is too toxic,and recruitment of patients to receivethis combination has beendiscontinued. By contrast, recruitmentinto treatment arms involving esca-lating dosages of gemcitabine plus carboplatinis expected to continue.ConclusionThe efficacy of gemcitabine as aradiosensitizer is strongly supportedby accumulating clinical data. Whenadministered together with radiationtherapy, gemcitabine is associatedwith favorable overall survival andresponse rates, and may be used inpatients with a low performance status.Although the safe weekly dose ofgemcitabine has yet to be determined,it appears to be at least 300 mg/m2.An ongoing clinical trial may soonestablish the maximum tolerated doseof gemcitabine to be used during radiationtherapy. Toxicity may be minimizedby using modern technologysuch as three-dimensional conformalradiotherapy. Additional clinical trialsare needed to assess the long-termefficacy and safety of gemcitabineadministration during radiotherapy.
Disclosures:
Dr. Choy has receivedgrant support from and served on speakers'bureaus for Eli Lilly.
References:
1.
Mattson K, Holsti LR, Holsti P, et al: Inoperablenon-small cell lung cancer: Radiationwith or without chemotherapy. Eur J CancerClin Oncol 24:477-482, 1988.
2.
Morton RF, Jett JR, McGinnis WL, et al:Thoracic radiation therapy alone comparedwith combined chemoradiotherapy for locallyunresectable non-small cell lung cancer. A randomized,phase III trial. Ann Intern Med115:681-686, 1991.
3.
Dillman RO, Herndon J, Seagren SL, etal: Improved survival in stage III non-smallcelllung cancer: Seven-year follow-up of cancerand leukemia group B (CALGB) 8433 trial.J Natl Cancer Inst 88:1210-1215, 1996.
4.
Le Chevalier T, Arriagada R, Quoix E, etal: Radiotherapy alone versus combined chemotherapyand radiotherapy in nonresectablenon-small-cell lung cancer: First analysis of arandomized trial in 353 patients. J Natl CancerInst 83:417-423, 1991.
5.
Kubota K, Furuse K, Kawahara M, et al:Role of radiotherapy in combined modalitytreatment of locally advanced non-small-celllung cancer. J Clin Oncol 12:1547-1552, 1994.
6.
Furuse K, Fukuoka M, Kawahara M, etal: Phase III study of concurrent versus sequentialthoracic radiotherapy in combination withmitomycin, vindesine, and cisplatin inunresectable stage III non-small-cell lung cancer.J Clin Oncol 17:2692-2699, 1999.
7.
Pierre F, Maurice P, Gilles R, et al: A randomizedphase III trial of sequentialchemoradiotherapy versus concurrentchemoradiotherapy in locally advanced nonsmallcell lung cancer (NSCLC) (GLOT-GFPCNPC 95-01 study) (abstract 1246). Proc Am SocClin Oncol 20:312a, 2001.
8.
Zatloukal PV, Petruzelka L, Zemanova M,et al: Concurrent versus sequentialradiochemotherapy with vinorelbine pluscisplatin in locally advanced non-small celllung cancer. A randomized phase II study (abstract1159). Proc Am Soc Clin Oncol 21:290a,2002.
9.
Choy H, Curran WJ, Scott CB, et al: Preliminaryreport of locally advancedmultimodality protocol (LAMP): ACR 427: Arandomized phase II study of three chemo-radiationregimens with paclitaxel, carboplatin,and thoracic radiation (TRT) for patients withlocally advanced non small cell lung cancer(LA-NSCLC) (abstract 1160). Proc Am SocClin Oncol 21:291a, 2002.
10.
Huber RM, Schmidt M, Flentje M, et al:for the BROCAT group. Induction chemotherapyand following simultaneous radio/chemotherapyversus induction chemotherapy andradiotherapy alone in inoperable NSCLC (stageIIIA/IIIB) (abstract 2501). Proc Am Soc ClinOncol 22:622, 2003.
11.
Curran WJ, Scott C, Langer R, et al:Long-term benefit is observed in a phase IIIcomparison of sequential vs concurrent chemoradiationfor patients with unresected stage IIINSCLC: RTOG 9410 (abstract 2499). Proc AmSoc Clin Oncol 22:621, 2003.
12.
Choy H: Combination chemoradiotherapywith gemcitabine: Potential applications.Oncology (Huntingt) 14(7 suppl4):20-25, 2000.
13.
Hertel LW, Boder GB, Kroin JS, et al:Evaluation of the antitumor activity ofgemcitabine (2â²,2â²-difluoro-2â²-deoxycytidine).Cancer Res 50:4417-4422, 1990.
14.
Shewach DS, Hahn TM, Chang E, et al:Metabolism of 2â²,2â²-difluoro-2â² deoxycytidineand radiation sensitization of human colon carcinomacells. Cancer Res 54:3218-3223, 1994.
15.
Lawrence TS, Chang EY, Hahn TM, etal: Delayed radiosensitization of human coloncarcinoma cells after a brief exposure to 2â²2â²-difluoro-2-deoxycytidine (gemcitabine). ClinCancer Res 3:777-782, 1997.
16.
Lawrence TS, Eisbruch A, McGinn CJ,et al: Radiosensitization by gemcitabine. Oncology13(suppl 5):55-60, 1999.
17.
Gemzar (gemcitabine), in Physicians’Desk Reference 2004, 58th ed, pp 1837-1842.Montvale, NJ, Thompson PDR, 2004.
18.
De Braud F, Pastorino U, Catalano G, etal: Chemotherapy followed by three-times dailyhyperfractionated accelerated radiotherapy instage IIIa (pN2)/IIIb non-small cell lung cancer(abstract 550P). Ann Oncol 11(suppl 4):121,2000.
19.
Isla D, Mayordomo JI, Cajal R, et al: Aphase II trial of high-dose gemcitabine (GEM)+ cisplatin (CDDP) and sequential radiotherapyfor unresectable stage III non small cell lungcancer (NSCLC); preliminary results (abstract1997). Proc Am Soc Clin Oncol 19:510a, 2000.
20.
Van Zandwijk N, Smit EF, Kramer GW,et al: Gemcitabine and cisplatin as inductionregimen for patients with biopsy-proven stageIIIA N2 non-small-cell lung cancer: A phase IIstudy of the European Organization for Researchand Treatment of Cancer Lung CancerCooperative Group (EORTC 08955). J ClinOncol 18:2658-64, 2000.
21.
Vinolas N, Casas F, Soler G, et al: Frontlinetreatment of advanced non-small-cell lungcancer (NSCLC) with alternating chemotherapy(CT) and concurrent radiotherapy (RT):Phase II (abstract 2134). Proc Am Soc ClinOncol 19:542a, 2000.
22.
Cicënas S, Pipirienë T, Burneckis A, etal: Gemcitabine-cisplatin (GC) versusetoposide-cisplatin (EC) in patients with inoperablestage IIIA/IIIB non-small cell lung cancer(NSCLC) with intermittent radiotherapy:A randomized phase II trial (abstract 2831).Proc Am Soc Clin Oncol 20:270b, 2001.
23.
Crino L, De Marinis F, Scagliotti G, etal: Neoadjuvant chemotherapy withgemcitabine and platinum in unresectable stageIII non-small cell lung cancer (NSCLC): Aphase II experience with a new schedule (abstract1311). Proc Am Soc Clin Oncol 20:329a,2001.
24.
Migliorino MR, De Marinis F, Nelli F,et al: A 3-week schedule of gemcitabine pluscisplatin as induction chemotherapy for stageIII non-small cell lung cancer. Lung Cancer35:319-327, 2002.
25.
Fogarty G, Ball D, Rischin D: Radiationrecall reaction following gemcitabine.Lung Cancer 33:299-302, 2001.
26.
Scalliet P, Goor C, Galdermans D, et al:Gemzar (gemcitabine) with thoracic radiotherapy-A phase II pilot study in chemonaivepatients with advanced non-small cell lung cancer(NSCLC) (abstract 1923). Proc Am Soc ClinOncol 17:499a, 1998.
27.
Gonzalez E, Sanchez-Rovira P, Jaen A,et al: Phase I study of concomitant gemcitabine(G) with radiotherapy (RT) in stage III NSCLC.Eur J Cancer 35(suppl 4):A1054, 1999.
28.
Zinner R, Fossella F, Komaki R, et al:Phase I trial of concurrent gemcitabine (G) pluschest radiotherapy (XRT), followed by consolidationsystemic chemotherapy with G pluscisplatin (CDDP), for patients with stage III andmedically inoperable stage II non-small celllung cancer (NSCLC) (abstract 1798). Proc AmSoc Clin Oncol 18:466a, 1999.
29.
Trodella L, Granone P, Valente S, et al:Phase I trial of weekly gemcitabine and concurrentradiotherapy in patients with inoperablenon-small-cell lung cancer. J Clin Oncol20:804-810, 2002.
30.
Trodella L, Cortesi E, D’Angelillo RM,et al: Concurrent radiochemotherapy withweekly gemcitabine in IIIAN2 non-small celllung cancer: Analysis of an ongoing phase IItrial (abstract 2713). Proc Am Soc Clin Oncol22:675, 2003.
31.
Vokes EE, Herndon JE 2nd, Crawford J,et al: Randomized phase II study of cisplatinwith gemcitabine or paclitaxel or vinorelbineas induction chemotherapy followed by concomitantchemoradiotherapy for stage IIIB nonsmall-cell lung cancer: Cancer and LeukemiaGroup B study 9431. J Clin Oncol 20:4191-4198, 2002.