Current Paradigms in First-Line Treatment of Non–Small-Cell Lung Cancer
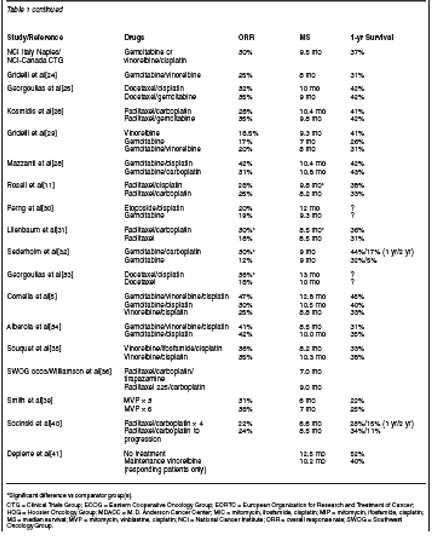
Standard first-line chemotherapy for the majority of patients withadvanced non–small-cell lung cancer (NSCLC) consists of platinumbasedcombination regimens including one of the newer-generationagents, such as gemcitabine (Gemzar), a taxane, vinorelbine(Navelbine), or irinotecan (Camptosar). Several effective regimens areavailable, the choice of which will depend on treatment goals, individualpatient or disease factors, as well as physician preferences. Thispaper surveys randomized trials of many of the newer-generation chemotherapycombinations in patients with advanced NSCLC to examineseveral issues, such as which new-generation regimen to use, whethera platinum agent is needed, the optimal number of drugs in the combination,and treatment duration.
ABSTRACT: Standard first-line chemotherapy for the majority of patients withadvanced nonâsmall-cell lung cancer (NSCLC) consists of platinumbasedcombination regimens including one of the newer-generationagents, such as gemcitabine (Gemzar), a taxane, vinorelbine(Navelbine), or irinotecan (Camptosar). Several effective regimens areavailable, the choice of which will depend on treatment goals, individualpatient or disease factors, as well as physician preferences. Thispaper surveys randomized trials of many of the newer-generation chemotherapycombinations in patients with advanced NSCLC to examineseveral issues, such as which new-generation regimen to use, whethera platinum agent is needed, the optimal number of drugs in the combination,and treatment duration.The majority of patients withnon-small-cell lung cancer(NSCLC) who are of good performancestatus will receive chemotherapyat some time in themanagement of their disease. A metaanalysisof 52 randomized trials, publishedin 1995, supported the use ofcisplatin-based chemotherapy inNSCLC, based on a 1.5-month improvementin median survival and a10% absolute 1-year survival benefitwith chemotherapy vs best supportivecare (NSCLC CollaborativeGroup).[1] Chemotherapy was alsoassociated with improved symptomcontrol and quality of life. More recently,platinum-based doublets containingnewer agents includinggemcitabine (Gemzar), the taxanes,vinorelbine (Navelbine), irinotecan(Camptosar) and others have demonstratedimproved activity in the firstlinetreatment of NSCLC, withresponse rates ≥ 40% in several randomizedtrials.[2-5]With several potential regimensavailable, it is worth examining keytrials that helped establish currenttreatment approaches in NSCLC. Thispaper will focus on first-line chemotherapyfor NSCLC, using mainly phase III randomized trials to examineissues such as which new-generationregimen to use, whether aplatinum agent should be included,and if so, cisplatin or carboplatin(Paraplatin), how many drugs to combine,and the optimal treatment duration.Trials that will be discussed,primarily in terms of response andsurvival results, are summarized inTable 1.Which Third-GenerationRegimen to Use?Vinorelbine/Platinum Trials
Vinorelbine was the first of thethird-generation chemotherapy drugsestablished for use in lung cancer, and has now accrued more than adecade of experience in this disease.Le Chevalier et al conducted a pivotaltrial comparing vinorelbine/cisplatin,vindesine/cisplatin, and vinorelbinealone in 612 patients with stage IIIA/Band IV NSCLC.[2] Response rate improvedsignificantly with vinorelbine/cisplatin compared with the othertreatments, (30%, 19% [P = .02], 14%[P < .001], respectively). Notably,this was the first trial demonstratinga 40% 1-year survival rate in advancedNSCLC (in the vinorelbine/cisplatin patients), whereas 1-yearsurvival rates with previous-generationregimens had ranged around 20%to 25%.In the TAX 326 trial, first-line vi-norelbine/cisplatin was compared withdocetaxel/cisplatin and docetaxel/carboplatinin more than 1,200 advancedNSCLC patients, with no significantbenefit shown for vinorelbine.[3] Depierreet al also compared vinorelbine/cisplatin with single-agentvinorelbine. Response rate was significantlyhigher (43% vs 16%, P =.0001) when cisplatin was added tosingle-agent vinorelbine, althoughthere were no differences in eithermedian survival (~ 8 mo) or 1-yearsurvival rates (~ 35%) between treatmentgroups in this study.[6]
Paclitaxel/Platinum Trials
An important study conducted bythe Eastern Cooperative OncologyGroup (ECOG 5592) helped establishthe role of paclitaxel in NSCLC.[7]Patients (N = 599) received first-lineetoposide/cisplatin or paclitaxel/cisplatin,with paclitaxel administered attwo doses (135 mg/m2 or 250 mg/m2)infused over 24 hours. The two paclitaxelgroups combined had superior1-year survival rates and median survivaltimes (39% and 9.9 months vs32% and 7.6 months, P = .048) andsignificantly improved response rates(~26% vs 12%, P < .001) comparedwith those achieved with etoposide/cisplatin, respectively.A European Organization for Researchand Treatment of Cancer(EORTC) trial with more than 300advanced NSCLC patients comparedteniposide (Vumon)/cisplatin and paclitaxel/cisplatin using a more standard3-hour paclitaxel infusion time.A significant response benefit wasseen in the paclitaxel arm (41% vs28%, P = .018), although 1-year survivalrates were similar (~42%) in thetwo groups.[4] In a study of first-linepaclitaxel/cisplatin vs cisplatin alonein 414 patients, Gatzemeier et al reporteda significant response improvement(26% vs 17%, P = .028) but nosurvival benefit with the paclitaxeldoublet.[8]Standard initial chemotherapy foradvanced NSCLC in the United Statesis paclitaxel plus carboplatin. Belaniet al[9] compared paclitaxel and carboplatinwith the second-generationregimen of etoposide and cisplatin,and showed only nonsignificant trendsin favor of the paclitaxel regimen.The Southwest Oncology Group(SWOG) compared paclitaxel/carboplatinand vinorelbine/cisplatin(SWOG 9509) and found responserates of 25% in the paclitaxel/carboplatinarm and 28% in the vinorelbine/cisplatin arm, with similarsurvival rates (median: 8 months).[10]Patients on the vinorelbine arm hadsignificantly more nausea and vomiting,whereas patients on the paclitaxelarm had significantly moreneurotoxicity. More patients receivingvinorelbine/cisplatin discontinuedtreatment because of toxicity, but quality-of-life analyses identified no differencesbetween the two arms.In a multinational study reported byRosell et al, 618 chemotherapy-naiveadvanced NSCLC patients receivedpaclitaxel infused over 3 hours combinedwith carboplatin or cisplatin, withno differences in response rates (~25%)shown.[11] However, with 22 monthsof follow-up, updated analysis showedmedian survivals of 8.2 and 9.8months, respectively (P = .019), suggestingthat cisplatin may be a slightlymore active agent than carboplatinwhen combined with paclitaxel.To compare the most importantthird-generation regimens in advancedNSCLC (stage IIIB, IV, or recurrentdisease), Schiller and colleagues atECOG undertook a large 1,200-patienttrial (ECOG 1594) that comparedthe best arm of their previous trial(paclitaxel at 135 mg/m2 infused over24 hours and cisplatin at 75 mg/m2) tothree other third-generation regimens,including gemcitabine/cisplatin, docetaxel/cisplatin, and paclitaxel at 225mg/m2 infused over 3 hours with carboplatin(Paraplatin). The results ofthe paclitaxel/carboplatin arm weresomewhat disappointing, with a responserate of only 17% and medianand 1-year survival rates of 8.5 monthsand 34%, respectively.[12] However,these results were similar to those seenin the other three arms, and none of the three study arms was significantlybetter than the reference arm of paclitaxeland cisplatin.Gemcitabine/Platinum Studies
Gemcitabine has been studied combinedwith platinum as first-line therapyfor NSCLC in several phase IIIrandomized studies. Sandler et al fromthe Hoosier Oncology Group (HOG)conducted a randomized phase IIIstudy (n = 522) comparing cisplatinat 100 mg/m2 on day 1 plus gemcitabineat 1,000 mg/m2 on days 1, 8, and15 every 4 weeks to cisplatin at 100mg/m2 on day 1, every 4 weeks.[13]Patients receiving cisplatin/gemcitabinehad significantly improved response(30.4% vs 11.1%; P < .0001),median time to progression (5.6 vs3.7 months; P = .0013), and overallsurvival (9.1 vs 7.6 months; P = .004).Toxicity was mostly hematologic(more pronounced in the combinationarm) with grade 4 neutropenia in35.3% of patients vs 1.2%; neutropenicfever was < 5% in both arms.Grade 4 thrombocytopenia occurredin 25.4% of patients on the combinationarm vs 0.8% of those on themonotherapy arm.In a study conducted by Cardenalet al comparing gemcitabine/cisplatinand the second- generation regimenetoposide/cisplatin in 133 advancedNSCLC patients, the gemcitabine regimenproduced a significantly higheroverall response rate (41% vs 22%,P = .02) and a significant delay intime to disease progression (6.9 vs4.3 months, P = .01).[14] Survivalwas similar in the two arms.The ECOG 1594 study mentionedpreviously, which compared fourthird-generation regimens, includedgemcitabine/cisplatin as one treatmentarm. Response and 1-year survivalrates with the gemcitabine combination(22%, 36%) were similar to thoseachieved with paclitaxel/cisplatin(21%, 31%), and higher than those ofdocetaxel/cisplatin (17%, 31%) andpaclitaxel/carboplatin (17%, 34%) althoughthese differences were notsignificant.[12] The only significantstudy finding was that of time to diseaseprogression, favoring the gemcitabine/cisplatin doublet (4.2 vs 3.4, 3.7,and 3.1 months, respectively, P < .05).The gemcitabine/cisplatin doubletand paclitaxel/cisplatin doublets comprisedtwo out the three arms of anEORTC trial (EORTC 08975) [15] vsMIC (mitomycin [Mutamycin], ifosfamide[Ifex], cisplatin). No significantdifferences were seen for eitherresponse or survival rates.Notwithstanding the excellent responserates achieved when gemcitabineis combined with cisplatin, manyphysicians prefer to use carboplatinbased on tolerability issues. Whenadministered with carboplatin, gemcitabineis generally given on a day 1,day 8 schedule and the carboplatindose must be reduced to an area underthe concentration curve (AUC) of5 to lessen risk of severe thrombocytopenia.Gemcitabine/carboplatin hasonly been compared in randomizedtrials to second-generation regimens.In one study of gemcitabine/carboplatinvs MIC or MVP (mitomycin,vinblastine, cisplatin), nosignificant differences in response andsurvival outcomes were noted.[16]However, a significant survival advantagefavoring gemcitabine/carboplatinover MIP (mitomycin,ifosfamide, cisplatin) was demonstratedin another randomized trial (n =422) for median survival, 10.0 vs 6.5mo; 1-year survival rates, 38% vs28%; P = .0043.[17] Gemcitabine/carboplatinalso provided significantlybetter response (27% vs 15%, P < .05) and mean (11.5 vs 7.9 mo, P =.0001) and 1-year (36% vs 12%, P < .05) survival results than the secondgenerationvinblastine/cisplatin regimen.[18] These results thusdemonstrate the utility of carboplatinas an alternate to cisplatin in gemcitabine-containing regimens.Docetaxel/Platinum Regimens
The four-arm ECOG 1594 studyincluded the docetaxel/cisplatin regimen,wherein the response rate (17%)and median survival (7.4 months)were lower than those achieved withthe paclitaxel/cisplatin or gemcitabine/cisplatin doublets (P = not significant).[12] On the other hand, in thelarge TAX 326 study, docetaxel/cisplatinhad improved response rates(32%, 24%, 25%, P = .029) relativeto docetaxel/carboplatin and vinorelbine/cisplatin, respectively.[3] Furthermore,while 2-year survival ratesare often not reported, docetaxel/cisplatintreatment resulted in a 7% absolute2-year survival improvementover that achieved with vinorelbine/cisplatin (21% vs. 14%). Quality-oflifeassessments also favored the docetaxelarms in this trial.In a smaller study from Japan involvingmore than 300 chemotherapy-naive stage IV NSCLC patients,docetaxel/cisplatin significantly improvedresponse rate (37% vs 21%,P < .01) and median survival (11.3 vs9.6 mo, P = .014) over that achievedwith vindesine/cisplatin, and there wasa trend toward improved 1-year survival(48% vs 41%).[19]Irinotecan/Platinum Regimens
Randomized trials of first-lineirinotecan in advanced NSCLC havealso been carried out. Negoro et alcompared irinotecan/cisplatin, vindesine/cisplatin, and irinotecan alonein approximately 400 patients.[20]Significant improvements in responserates (44%, 32%, 21%, respectively,PP = .004, for irinotecan/cisplatinvs vindesine/cisplatin; and 42.1vs 36.4 weeks for irinotecan alone vsvindesine/cisplatin, P = .018). In contrast,preliminary data from a randomizedtrial comparing irinotecan/cisplatin with vindesine/cisplatin, paclitaxel/carboplatin, and gemcitabine/cisplatin showed similar response rates(~30%) for the four treatment groups,with no survival data yet available.[21]Pemetrexed
Pemetrexed (Alimta) is a multitargetedantifolate with response rates of≥ 20% as a single agent and approximately40% when combined with cisplatin,even in patients with advancedNSCLC.[22,23] This agent has demonstratedits activity in second-lineNSCLC, and randomized trials in thefirst-line setting are awaited to determine further the role of pemetrexed.Possible combination partners underinvestigation include gemcitabine, vinorelbine,carboplatin, and oxaliplatin(Eloxatin).Is Platinum Necessary?Data from several randomized trialsof first-line treatment in advancedNSCLC can be used to address whethera platinum agent is necessary. Forexample, EORTC 08975 includedcombinations of cisplatin withgemcitabine or paclitaxel and the nonplatinumpaclitaxel/gemcitabine doublet.[15] No significant differenceswere noted, although the lowest responserate of 27% and median survivaltime of 6.9 months occurred inthe nonplatinum arm-results lowerthan one would expect with a thirdgenerationplatinum-based regimen.A trial by Gridelli et al undertakenby the National Cancer Institute (NCI)of Italy Naples group and the NCICanada also found inferior results forthe non-platinum regimen (vinorelbine/gemcitabine) compared withgemcitabine or vinorelbine combinedwith cisplatin.[24] Time to diseaseprogression was significantly poorerin the nonplatinum arm (P = .004),while response and 1-year survivaldifferences were not statistically significant,although trended in favor ofcisplatin use. Toxicity was greater inthis study in the cisplatin arm, butpatients treated with the cisplatinbasedregimens had more improvementin their symptoms. It is also ofinterest that, despite greater toxicityin the platinum arm, quality of lifewas similar in both arms.Two other studies involving approximately900 chemotherapy-naiveadvanced NSCLC patients demonstratedvirtual equivalence of nonplatinumand platinum regimens, incomparisons of docetaxel/cisplatin vsdocetaxel/gemcitabine[25], and paclitaxel/carboplatin vs paclitaxel/gemcitabine.[26] Response rates ranged from28% to 35% with the four regimens,with ~10-month median survival timesand ~40% 1-year survival rates.While these study results are somewhatmixed regarding the need forplatinum therapy, a recent meta-analysis by D'Addario et al provides supportfor platinum-containing chemotherapy.[27] This meta-analysis comparedeffects of platinum and nonplatinumregimens administered tomore than 7,500 patients with NSCLCparticipating in 37 trials. Responserates significantly improved with platinum-based chemotherapy (P < .0001)when all trials were considered, andalso when only the 1,563 patientstreated with third-generation regimenswere assessed (P = .0032). Survivalalso favored cisplatin-based treatment,although the difference was not statisticallysignificant for the third-generationregimens.Cisplatin or Carboplatin?Only a few randomized trials ofthird-generation regimens for NSCLCdirectly compared cisplatin and carboplatin.Paclitaxel/cisplatin and paclitaxel/carboplatin[12] (althoughwith different paclitaxel doses and infusiontimes) made up two of the fourtreatment arms in ECOG 1594.[12]No significant differences in responseor survival were seen in these trials.Rosell et al compared paclitaxel infusedover 3 hours combined withcarboplatin or cisplatin. Althoughthere were no differences in responserates (~25%) shown,[11] median survivalfavored cisplatin (8.2 vs9.8 mo, P = .019), suggesting thatcisplatin may be a slightly more activeagent than carboplatin whencombined with paclitaxel.Very similar results were seen intwo of the arms of the TAX 326 studyin which docetaxel at 75 mg/m2 wascombined with either cisplatin or carboplatin.Response rates for the cisplatinand carboplatin arms were 32%and 24%, respectively, with survivalsof 11.3 and 10.4 months.[3] The studywas not designed to compare thetwo docetaxel arms to each other, soP values are not available. Finally,combinations of gemcitabine with cisplatinor carboplatin were assessed byMazzanti et al,[28] who found responserates of 42% vs 31%, respectively(P = not significant), andvirtually equivalent median and 1-yearsurvivals (~10 months and 42%, respectively).Thus, while no significant differencesbetween cisplatin and carboplatinhave been demonstrated in mosttrials, response rates and survivalshave usually been somewhat higheroverall with cisplatin. Based on thesefindings, substitution of cisplatin withcarboplatin may be acceptable in thepalliative setting of advanced diseaseto reduce toxicity risk, whereas cisplatinmay be more appropriately appliedin the curative setting (ie, asneoadjuvant or adjuvant therapy) inattempts to derive the greatest potentialtherapeutic benefit.How Many Drugsin the Regimen?Several randomized trials of firstlinechemotherapy for NSCLC addressthe issue of the optimal numberof drugs. For example, in the trial byLeChevalier et al,[2] response ratedoubled when cisplatin was added tosingle-agent vinorelbine therapy(30% vs 14%, P < .001), with a nonsignificanttrend toward improvedsurvival (7.8 vs 10 months mediansurvival, and 32% vs 40% 1-year survivalrate). However, another trialcomparing vinorelbine alone with vinorelbine/cisplatin also found a significantresponse benefit for thetwo-drug regimen (43% vs 16%, P =.0001), but survival results were similar.[6] When elderly NSCLC patientsreceived single-agent vinorelbine orgemcitabine or the combination ofthose agents, Gridelli et al noted noresponse or survival differences betweentreatment groups.[29]Finally, a phase II randomizedstudy comparing the second-generationregimen etoposide/platinum withgemcitabine alone resulted in similarresponse and survival data, althoughgemcitabine tolerability was superiorin these patients.[30] In contrast, someevaluations of third-generation combinationsshowed significant improvementsin both response andsurvival, for example, when carboplatinwas added to paclitaxel[31] orto gemcitabine,[32] or with cisplatinadded to docetaxel.[33] Randomizedtrials assessing three-drug regimensin NSCLC demonstrated no responseor survival benefits over two-drug combinations, and increased toxicitythat sometimes required early treatmentdiscontinuation.[5,34-36]Data from meta-analyses are alsoinformative regarding how manydrugs to use. In a meta-analysis publishedby Lilenbaum et al, two-drugchemotherapy regimens demonstrateda nearly twofold increase in responserate over single-agent therapyin patients with advanced NSCLC,while 1-year survival results weresimilar.[37] Another meta-analysis reportedby Delbaldo et al derived datafrom more than 14,500 advancedNSCLC patients involved in 65 trials.[38] Two drugs significantly enhancedresponse and survival overthat achieved with one drug (P < .001), while three drugs added to response(P < .001) but not to survivaland also increased toxicity (P = .95).Duration of TherapyA small number of trials have assessedoptimal chemotherapy durationfor NSCLC patients. In a trialcomparing MVP (mitomycin, vinblastine,cisplatin) * 3 courses vs MVP *6, response rate was slightly higherwith more treatment (38% vs 31%)but survival was similar.[39] Anothertrial used paclitaxel/carboplatin forfour cycles vs continuing until diseaseprogression, and while no improvementsin response or survivalwere seen at the median, 1-year, or2-year follow-ups, longer treatmentduration resulted in a significant increasein severe neurotoxicity.[40]In another study, patients respondingto initial treatment were randomlyassigned to receive maintenancevinorelbine or no additional treatment.Survival results were poorer inthe maintenance group because oftreatment-related toxic deaths.[41]Overall, results of these trials thussupport administration of approximatelythree to four chemotherapycourses rather than longer treatmentperiods.ConclusionBased on the randomized trials discussedherein, platinum-based combinationchemotherapy regimens that include third-generation agents, suchas gemcitabine, paclitaxel, docetaxel,vinorelbine, or irinotecan, modestlyimprove outcome of patientswith advanced NSCLC comparedwith previous generation regimens,although it seems that a new survivalplateau has been reached in this disease.Carboplatin may be slightly lessactive than cisplatin but is better toleratedand thus may be preferred inthe palliative setting.At this point, the data do not indicatethat nonplatinum chemotherapyregimens should be preferred overthose containing platinum based onsuperiority. Nevertheless, when patientscannot tolerate a platinumbasedcombination (ie, elderly or poorperformance status), a nonplatinumdoublet may provide more benefitthan single-agent treatment. In the2003 American Society ofClinical Oncology updated guidelinesfor treatment of unresectableNSCLC, Pfisters and the Expert Panelrecommended a change from previousguidelines indicating thatnon-platinum-containing chemotherapyregimens may be used as alternativesto platinum-based combinationsin the first line for stage IVNSCLC.[42]Results also show that continuingtreatment beyond four to six cyclesmay result in unacceptable toxicitywith an increase in either response orsurvival. Thus, this brief survey ofrandomized trials supports the currentfirst-line chemotherapy approachin advanced NSCLC, which is withfour to six cycles of a platinumcontainingdoublet. Finally, clinicalissues including sequencing of treatments,second- and third-line therapies,chemotherapy combinations withnovel agents administered either concurrentlyor sequentially, toxicities,quality of life, and treatment costs,which were beyond the scope of thispaper, are additional important considerationsfor patients with NSCLC.Interestingly, Schiller et al, in reportingthe results of ECOG 1594 comparingfour chemotherapy regimens for advanced NSCLC, noted that toxicitywas generally similar for the regimens.[12] However, based on thisand other trials, it was unclear whetherreductions in toxicity improve qualityof life.[43]Since no standard front-line combinationin NSCLC has demonstratedsuperiority, any of the thirdgenerationplatinum-based doubletsis considered acceptable therapy. Thechoice of regimen may be based ontoxicity profiles, convenience, andperhaps even cost.
Disclosures:
Dr. Shepherd hasacted as a consultant for and received honoriariafrom Eli Lilly, Aventis, and GlaxoSmithKline.
References:
1.
Non-small Cell Lung Cancer CollaborativeGroup. Chemotherapy in non-small celllung cancer: Meta-analysis using updated datain individual patients from 52 randomized clinicaltrials. Br Med J 311:899-909, 1995.
2.
Le Chevalier T, Brisgand D, Douillard JY,et al: Randomized study of vinorelbine andcisplatin versus vindesine and cisplatin versusvinorelbine alone in advanced non-small celllung cancer: Results of a European multicentertrial including 612 patients. J Clin Oncol12:360-367, 1994.
3.
Fossella F, Pereira JR, von Pawel J, et al:Randomized multinational phase III study ofdocetaxel plus platinum combinations versusvinorelbine plus cisplatin for advanced nonsmallcell lung cancer: The TAX 326 StudyGroup. J Clin Oncol 21:3016-3024, 2003.
4.
Giaccone G, Splinter TAW, DeBruyne C,et al, for the EORTC Lung Cancer CooperativeGroup: Randomized study of paclitaxelcisplatinversus cisplatin-teniposide in patientswith advanced non-small cell lung cancer. JClin Oncol 16:2133-2141, 1998.
5.
Comella P, Frasci G, Panza N, et al: Randomizedtrial comparing cisplatin,gemcitabine, and vinorelbine with eithercisplatin and gemcitabine or cisplatin andvinorelbine in advanced non-small cell lungcancer: interim analysis of a phase III trial ofthe Southern Italy Cooperative OncologyGroup. J Clin Oncol 18:1451-1457, 2000.
6.
Depierre A, Chastang C, Quoix E, et al:Vinorelbine versus vinorelbine plus cisplatinin advanced non-small cell lung cancer: A randomizedtrial. Ann Oncol 5:37-42, 1994.
7.
Bonomi PB, Kim K, Fairclough D, et al:Comparison of survival and quality of life inadvanced non-small cell lung cancer patientstreated with two dose levels of paclitaxel combinedwith cisplatin versus etoposide withcisplatin: results of an Eastern CooperativeOncology Group trial. J Clin Oncol 18:623-631, 2000.
8.
Gatzemeier U, von Pawel J, Gottfried M,et al: Phase III comparative study of high-dosecisplatin versus a combination of paclitaxel andcisplatin in patients with advanced non-smallcell lung cancer. J Clin Oncol 18:3390-3399,2000.
9.
Belani CP, Natale RB, Lee JS, et al: Randomized phase III trial comparing cisplatin/etoposide versus carboplatin/paclitaxel in advancedand metastatic non-small cell lung cancer(NSCLC). Proc Am Soc Clin Oncol17:455a, 1998.
10.
Kelly K, Crowley J, Bunn PA Jr, et al:Randomized phase III trial of paclitaxel pluscarboplatin versus vinorelbine plus cisplatin inthe treatment of patients with advanced nonsmallcell lung cancer: A Southwest OncologyGroup Trial. J Clin Oncol 19:3210-3218, 2001.
11.
Rosell R, Gatzemeier U, Betticher DC,et al: Phase III randomized trial comparingpaclitaxel/carboplatin with paclitaxel/cisplatinin patients with advanced non-small cell lungcancer: a cooperative multinational trial. AnnOncol 13:1539-1549, 2002.
12.
Schiller JH, Harrington D, Belani C, etal, for the Eastern Cooperative OncologyGroup: Comparison of four chemotherapy regimensfor advanced non-small cell lung cancer(abstract 1751). N Engl J Med 346:92-98, 2002.
13.
Sandler AB, Nemunaitis J, Denham C,et al: Phase III trial of gemcitabine plus cisplatinversus cisplatin alone in patients with locallyadvanced or metastatic non-small-cell lungcancer. J Clin Oncol 18:122-130, 2000.
14.
Cardenal F, Lopez-Cabrerizo MP, AntonA, et al: Randomized phase III study ofgemcitabine-cisplatin versus etoposidecisplatinin the treatment of locally advancedor metastatic non-small cell lung cancer. J ClinOncol 17:12-18, 1999.
15.
Smit EF, van Meerbeeck J, Lianes P, etal: Three-arm randomized study of twocisplatin-based regimens and paclitaxel plusgemcitabine in advanced non-small cell lungcancer: A phase III trial of the European Organizationfor Research and Treatment of CancerLung Cancer Group (EORTC 08975). J ClinOncol 21:3909-3917, 2003.
16.
Danson S, Middleton MR, O’Byrne KJ,et al: Phase III trial of gemcitabine andcarboplatin versus mitomycin, ifosfamide, andcisplatin or mitomycin, vinblastine, andcisplatin in patients with advanced nonsmallcell lung carcinoma. Cancer 98:542-553, 2003.
17.
Rudd RM, Gower NH, James LE, et al:Phase III randomized comparison ofgemcitabine and carboplatin (GC) with mitomycin,ifosfamide and cisplatin (MIP) in advancednon-small cell lung cancer (NSCLC)(abstract 1164). Proc Am Soc Clin Oncol21:292a, 2002.
18.
Grigorescu AC, Draghici IN, Nitipir C,et al: Gemcitabine and carboplatin versuscisplatin and vinblastine in advanced non-smallcell lung cancer (NSCLC) stages III and IV: Aphase III randomized trial. Lung Cancer 37:9-14, 2002.
19.
Kubota K. Watanabe K, Kunitoh H, etal: Phase III randomized trial of docetaxel pluscisplatin versus vindesine plus cisplatin in patientswith stage IV non-small cell lung cancer:The Japanese Taxotere Lung Cancer StudyGroup. J Clin Oncol 22:254-261, 2004.
20.
Negoro S, Masuda N, Takada Y, et al,for the CPT-11 Lung Cancer Study Group West:Randomised phase III trial of irinotecan combinedwith cisplatin for advanced non-small celllung cancer. Br J Cancer 88:335-341, 2003.
21.
Ohe Y, Saijo N, Ohashi Y, et al, for the FACS Cooperative Group: Preliminary resultsof the Four-Arm Cooperative Study (FACS) foradvanced non-small cell lung cancer in Japan(abstract 2509). Proc Am Soc Clin Oncol22:624, 2003.
22.
Molina JR, Adjei AA: The role ofpemetrexed (Alimta, LY231514) in lung cancertherapy. Clin Lung Cancer 5:21-27, 2003.
23.
Shepherd FA, Arnold A, Neville A, etal: Phase II study of ALIMTA (PemetrexedDisodium, LY231514, MTA) and cisplatin asfirst-line therapy in patients with advanced nonsmallcell lung cancer (NSCLC). A study ofthe National Cancer Institute of Canada ClinicalTrials Group. Cancer 92:595-600, 2001.
24.
Gridelli C, Gallo C, Shepherd FA, et al:Gemcitabine plus vinorelbine compared withcisplatin plus vinorelbine or cisplatin plusgemcitabine for advanced non-small cell lungcancer: A phase III trial of the Italian GEMVINInvestigators and the National Cancer Instituteof Canada Clinical Trials Group. J Clin Oncol21:3025-3034, 2003.
25.
Georgoulias V, Papadakis E,Alexopoulos A, et al, for the Greek OncologyCooperative Group (GOCG) for Lung Cancer:Platinum-based and non-platinum-based chemotherapyin advanced non-small-cell lungcancer: a randomized multicenter trial. Lancet357:1478-1484, 2001.
26.
Kosmidis P, Mylonakis N, Nicolaides C,et al: Paclitaxel plus carboplatin versusgemcitabine plus paclitaxel in advanced nonsmallcell lung cancer: A phase III randomizedtrial. J Clin Oncol 20:3578-3585, 2002.
27.
D’Addario G, Pintilie M, Cerny T, et al:Platinum-based versus non-platinum-basedchemotherapy in advanced non-small cell lungcancer (NSCLC): A meta-analysis of the publishedliterature. Lung Cancer 41:68, 2003.
28.
Mazzanti P, Massacesi C, Rocchi MB,et al: Randomized, multicenter, phase II studyof gemcitabine plus cisplatin versusgemcitabine plus carboplatin in patients withadvanced non-small cell lung cancer. LungCancer 41:81-89, 2003.
29.
Gridelli C, Perrone F, Gallo C, et al: Chemotherapyfor elderly patients with advancednon-small cell lung cancer: The MulticenterItalian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J NatlCancer Inst 95:362-372, 2003.
30.
Perng RP, Chen YM, Liu JM, et al:Gemcitabine versus the combination ofcisplatin and etoposide in patients with inoperablenon-small cell lung cancer in a phase IIrandomized study. J Clin Oncol 15:2097-2102,1997.
31.
Lilenbaum RC, Herndon J, List M, etal: Single-agent (SA) versus combination chemotherapy(CC) in advanced non-small celllung cancer (NSCLC): A CALGB randomizedtrial of efficacy, quality of life (QOL), and costeffectiveness(abstract 2). Proc Am Soc ClinOncol 21:1a, 2002.
32.
Sederholm C: Gemcitabine (G) comparedwith gemcitabine plus carboplatin (GC)in advanced non-small cell lung cancer(NSCLC): A phase III study by the SwedishLung Cancer Study Group (SLUSG) (abstract1162). Proc Am Soc Clin Oncol 21:291a, 2002.
33.
Georgoulias V, Pallis AG, Kourousis C,et al: Docetaxel versus docetaxel/cisplatin inpatients with advanced non-small cell lung cancer:Preliminary analysis of a multicenter, randomizedphase III study. Clin Lung Cancer4:288-293, 2003.
34.
Alberola V, Camps C, Provencio M, etal: Cisplatin plus gemcitabine versus acisplatin-based triplet versus nonplatinum sequentialdoublets in advanced non-small celllung cancer: A Spanish Lung Cancer Groupphase III randomized trial. J Clin Oncol21:3207-3213, 2003.
35.
Souquet PJ, Tan EH, Rodrigues PereiraJ, et al: GLOB-1: A prospective randomizedclinical phase III trial comparing vinorelbinecisplatinwith vinorelbine-ifosfamide-cisplatinin metastatic non-small cell lung cancer patients.Ann Oncol 14:347, 2003.
36.
Williamson SK, Crowley JJ, Lara PN, etal: S0003: Paclitaxel/carboplatin (PC) v PC +tirapazamine (PCT) in advanced non-small celllung cancer (NSCLC): A phase III SouthwestOncology Group (SWOG) trial (abstract 2502).Proc Am Soc Clin Oncol 22:622, 2003.
37.
Lilenbaum RC, Langenberg P, DickersinK: Single agent versus combination chemotherapyin patients with advanced non-smallcell lung carcinoma: A meta-analysis of response,toxicity, and survival. Cancer 82:116-126, 1998.
38.
Delbaldo C, Syz N, Michiels S, et al:Adding a second or a third drug to a chemotherapyregimen in patients with advanced nonsmallcell lung carcinoma (NSCLC): A metaanalysisof the literature. Proc Am Soc ClinOncol 22:623 (abstract 2507), 2003.
39.
Smith IE, O’Brien ME, Talbot DC, etal: Duration of chemotherapy in advanced nonsmallcell lung cancer: A randomized trial ofthree versus six courses of mitomycin, vinblastine,and cisplatin. J Clin Oncol 19:1336-1343,2001.
40.
Socinski MA, Schell MJ, Peterman A,et al: Phase III trial comparing a defined durationof therapy versus continuous therapy followedby second-line therapy in advancedstageIIIB/IV non-small cell lung cancer. J ClinOncol 20:1335-1343, 2002.
41.
Depierre A, Quoix E, Mercier M, et al:Maintenance chemotherapy in advanced nonsmallcell lung cancer (NSCLC): A randomizedstudy of vinorelbine versus observation in patientsresponding to induction therapy (FrenchCooperative Oncology Group) (abstract 1231).Proc Am Soc Clin Oncol 20:309a, 2001.
42.
Pfisters DG, Johnson DH, Azzoli CG, etal: American Society of Clinical Oncologytreatment of unresectable non-small-cell lungcancer guideline: Update 2003. J Clin Onco.22:330-353, 2003.
43.
Ramsey SD, Moinpour CM, Lovato LC,et al: An economic analysis of southwest oncologygroup trial S9509: Cisplatin/vinorelbinevs. carboplatin/paclitaxel for advanced nonsmallcell lung cancer (abstract 1913). Proc AmSoc Clin Oncol 19:489a, 2000.
44.
Zinner R, Obasaju CK, Fossella FV, etal: Alimta plus carboplatin (AC) in patients (pts)with advanced non-small-cell lung cancer(NSCLC): A phase II trial (abstract 2580). ProcAm Soc Clin Oncol 22:642, 2003.
45.
Scagliotti G, Kortsik C, Castellano D, etal: Phase II randomized study of pemetrexed +carboplatin or oxaliplatin as front-line chemotherapyin patients with locally advanced ormetastatic non-small cell lung cancer (abstract2513). Proc Am Soc Clin Oncol 22:625, 2003.