Pemetrexed in Advanced NSCLC: A Review of the Clinical Data
The novel multitargeted antimetabolite pemetrexed (Alimta), recentlyapproved by the US Food and Drug Administration for the treatment ofmesothelioma when combined with cisplatin, is also active in first- andsecond-line non–small-cell lung cancer (NSCLC). In a phase III trialcomparing single-agent pemetrexed vs docetaxel (Taxotere) as secondlinetherapy in advanced NSCLC, survival was shown to be comparablebetween these agents, but side effects were significantly less frequentand severe for patients who received pemetrexed. In the frontlinesetting, phase II studies have shown significant activity and a veryfavorable toxicity profile of the combination of pemetrexed with a platinumagent. Pemetrexed has been well tolerated at systemic doses as aradiosensitizer when given as concurrent chest radiation, and a phaseI study is under way to assess its tolerability in combination withcarboplatin (Paraplatin) in this setting. Pemetrexed is an importantaddition to the armamentarium of medicines used to treat thoracicmalignancies, and merits study in combination with other drugs havingnovel mechanisms of action.
ABSTRACT: The novel multitargeted antimetabolite pemetrexed (Alimta), recentlyapproved by the US Food and Drug Administration for the treatment ofmesothelioma when combined with cisplatin, is also active in first- andsecond-line nonâsmall-cell lung cancer (NSCLC). In a phase III trialcomparing single-agent pemetrexed vs docetaxel (Taxotere) as secondlinetherapy in advanced NSCLC, survival was shown to be comparablebetween these agents, but side effects were significantly less frequentand severe for patients who received pemetrexed. In the frontlinesetting, phase II studies have shown significant activity and a veryfavorable toxicity profile of the combination of pemetrexed with a platinumagent. Pemetrexed has been well tolerated at systemic doses as aradiosensitizer when given as concurrent chest radiation, and a phaseI study is under way to assess its tolerability in combination withcarboplatin (Paraplatin) in this setting. Pemetrexed is an importantaddition to the armamentarium of medicines used to treat thoracicmalignancies, and merits study in combination with other drugs havingnovel mechanisms of action.
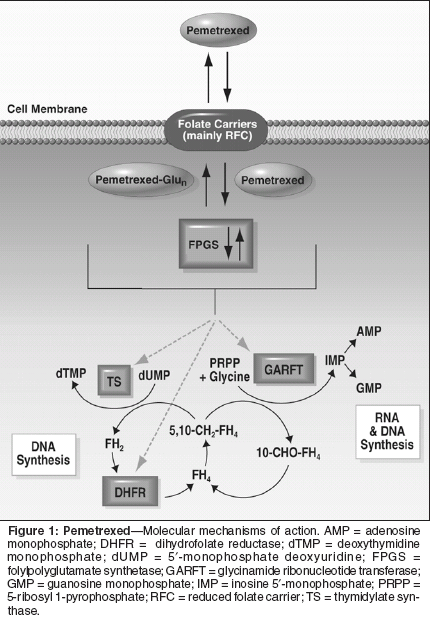
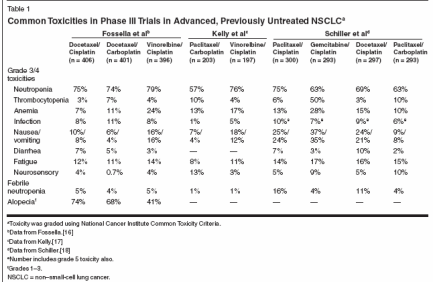
Lung cancer is the leading causeof cancer death in both menand women in the United States,with an estimated 173,770 new casesand 160,440 deaths in 2004.[1] Eightypercent of lung cancers are non-smallcelllung cancer (NSCLC). More peopledie from lung cancer than frombreast, prostate, and colon cancer combined,despite advances in the controlof local disease. This is partly becauseone-third of patients with lungcancer present with metastatic disease,but also because most patients withstage III and as many as 50% withearly-stage disease relapse after definitivetherapy.[2]A large meta-analysis in 1995showed a 10% increase in 1-year survivalrate and an improvement of 1.5months in median overall survival forcisplatin-containing regimens in advancedNSCLC.[3] In the late 1990s,third-generation agents including paclitaxel,docetaxel (Taxotere), gemcitabine(Gemzar), irinotecan (Camptosar), andvinorelbine (Navelbine) have been extensivelystudied. Combined with platinumagents, these form the standardof care for newly diagnosed advancedNSCLC. They produce response ratesof 10% to 20% in previously untreatedpatients as single agents[4-7] andin combination with platinum, 20%to 40% response and median survivalof 8 to 11 months in phase III trials.[8-13] However, these gains in survivalhave been rather modest.Moreover, there is no clearly demonstratedsuperiority of one of thesemodern platinum doublets over another.[14-18]Chemotherapy, in particular docetaxel,offers a survival advantage assecond-line therapy as well.[19,20]Gefitinib (Iressa) is indicated for usein patients whose tumors have progressedafter both platinum- and docetaxel-based chemotherapies[21,22]Pemetrexed (Alimta) has more recentlybeen introduced into clinicalstudy.[23] It has activity in multiplecancer types and is a novel antimetabolite.[24] On the basis of showinga survival advantage in combinationwith cisplatin as compared to singleagentcisplatin in mesothelioma, pemetrexedwas approved for use inmesothelioma by the US Food andDrug Administration (FDA).[25] As a single agent in the treatment of second-line NSCLC, it showed equal efficacywith improved tolerabilitycompared with docetaxel in a phaseIII study.[26] There are also data fromphase II platinum/pemetrexed doubletstudies showing efficacy comparableto best historical controls but withvery promising toxicity profiles (Table1). This article will focus on pemetrexedin the treatment of advancedNSCLC.PemetrexedPemetrexed interferes with at leastthree enzymes in pyrimidine and purinebiosynthesis, thymidylate synthase,dihydrofolate reductase, andglycinamide ribonucleotide formyltransferase(Figure 1).[27] A retrospectiveanalysis of severe adverseevents and their relationship to elevatedplasma homocysteine (a surrogatefor folate nutritional status) andmethylmelonic acid (a surrogate forvitamin B12 status) levels was performedduring the early clinical developmentof pemetrexed.[28]Elevated plasma homocysteine andmethylmelonic acid levels, indicatingfolate and B12 deficiency, were associatedwith a statistically significantlygreater risk of neutropenia, thrombocytopenia,nonhematologic toxicitiessuch as infection, mucositis, and diarrhea,and death.
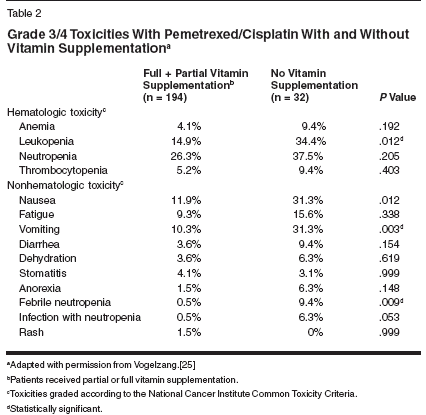
In December 1999, all patients beingtreated with pemetrexed beganreceiving folic acid to reduce homocysteineand vitamin B12 to reducemethylmelonic acid. This interventionhas since been shown to reduce homocysteineand methylmelonic acidplasma levels, and patients who receivedsupplementation in this wayexperienced significantly fewer toxicities.[33] This was shown quiteclearly through the phase III malignantpleural mesothelioma trial, wherethere was a clear improvement in thetolerability profile without a compromisein response or survival with theinitiation of mandatory vitamin supplementationpart of the way throughthe trial (Table 2).[25]Clinical Trials DataSingle-Agent PemetrexedWith and Without VitaminSupplementation: Phase I Trials
Multiple schedules of single agentpemetrexed have been evaluated inphase I trials, including a daily * 5schedule every 3 weeks,[30] weeklyfor 4 weeks every 4 weeks,[31] andonce every 3 weeks.[32] The scheduleof pemetrexed administered onceevery 3 weeks as a 10-minute intravenousbolus has been studied most extensively.In one study, 37 patientsreceived doses ranging from 50 to700 mg/m2 every 3 weeks.[32] However,neither folate nor vitamin B12supplementation was offered and sideeffects at the 700 mg/m2 dose levelwere significant.
Neutropenia, thrombocytopenia,mucositis, fatigue, diarrhea, rash,and/or anorexia were dose-limiting.Side effects including grade 3 neutropenia,thrombocytopenia, and cumulativeasthenia were experienced atthe preceding dose level of 600mg/m2, and this dose was consideredan acceptable one for further phase IItrials. There was activity against advancedpancreatic cancer and colorectalcancer on this every-3-weekschedule.Hammond[33] evaluated pemetrexedas a 10-minute intravenous bolusevery 3 weeks at a starting dose of600 mg/m2 plus oral folic acid in patientswho had had prior treatment inanother phase I trial. Doses rangedfrom 600 to 1,400 mg/m2 in 70 patients,all with prior chemotherapy.Neutropenia, anemia, and thrombocytopenia(more severe in the heavilypretreated patients), as well as nonhematologicside effects of rash, asthenia,pedal edema, and decline increatinine clearance, have been observed.Less toxicity was observedwith vitamin supplementation. More over, even at doses as high as 1,200mg/m2, none of these toxicities weredose-limiting. There was a partial responsein one patient with metastaticcolon cancer.Single-Agent Pemetexed WithoutVitamin Supplementation:Phase II Studies
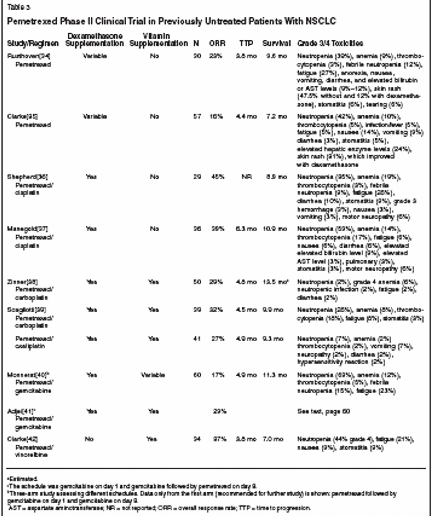
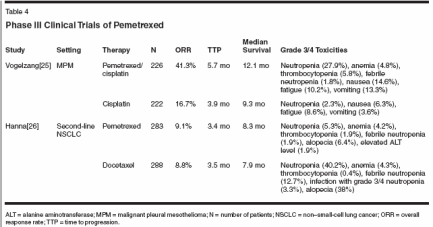
Two phase II single-agent studiesof pemetrexed were conducted in previouslyuntreated patients withNSCLC before the vitamin supplementationrequirement: one in Canadaand the other in Australia and SouthAfrica. Prophylactic dexamethasonewas not uniformly used in either study.The study done in Canada enrolled 33patients.[34] The starting pemetrexeddose was reduced from 600 to 500mg/m2 once every 21 days after threepatients had enrolled, because of excessivetoxicities in these patients.Grade 3/4 hematologic toxicitieswere as follows: neutropenia, 39%;febrile neutropenia, 12%; anemia,9%; and thrombocytopenia, 3%.Grade 3 nonhematologic toxicities(there were no grade 4 toxicities)were 39% for skin rash and 27% forfatigue. The incidences of anorexia,nausea, vomiting, diarrhea, andincreased bilirubin or aspartate aminotransferase(AST) levels were all9% to 12%. Grade 3 skin rash wasseen in 47.5% of patients not treatedwith dexamethasone and 12% ofpatients who received dexamethasoneprophylaxis. Response rate was23.3%, median overall survival was9.6 months, and the 1-year survivalrate was 25.3%.The study authored by Clarke etal done in Australia and South Africaused 600 mg/m2 of pemetrexedonce every 21 days throughout thestudy.[35] A total of 59 patients receivedtherapy. Response rate was15.8%, median time to progressionwas 4.4 months, median survivalwas 7.2 months, and the 1-year survivalrate was 32%. Grade 3/4hematologic toxicities included neutropenia (42%), anemia (10%), andthrombocytopenia (5%), and grade3/4 nonhematologic toxicities wereskin rash (31%) that improved afterdexamethasone prophylaxis was introduced,fatigue (5%), nausea(14%), vomiting (9%), infection/fever(5%), diarrhea (3%), and stomatitis(5%). Grade 3/4asymptomatic reversible hepatic enzymeelevation, which was seen in24% of patients, did not require dosereduction or delay (see Tables 3 and4).[25,26,34-42]Single-Agent Pemetrexed inPreviously Treated PatientsWith and Without VitaminSupplementation:Phase II and III Studies
There have been two studies ofsingle-agent pemetrexed for previouslytreated NSCLC: a phase II singlearmstudy by Smit et al[43] and aphase III study comparing pemetrexedwith docetaxel reported by Hanna etal.[26] In both studies pemetrexed wasdosed at 500 mg/m2 every 21 days.The phase II study did not includevitamin supplementation; the phaseIII study did. In both studies, all patientsreceived premedication withdexamethasone to reduce rash.Of 81 advanced NSCLC patientswho progressed within 3 months oftheir last chemotherapy in the phaseII study, the response rate was 8.9%,median time to progression was 2months, and median overall survivalwas 5.7 months. Grade 3/4 hematologictoxicities were neutropenia in35% of patients, anemia in 13%, andthrombocytopenia in 15%; grade 3/4nonhematologic toxicities were skinrash in 5%, lethargy in 5%, nausea in1%, vomiting in 3%, aminotransferaselevel elevation in 6%, and alkalinephosphatase level elevation in 1%.There was greatly decreased skin toxicitywith dexamethasone premedicationeven as compared with the studiesmentioned earlier that did not routinelyuse dexamethasone. Four deathson the study were possibly treatment related.In the study by Hanna et al,[26]patients were randomized to receivepemetrexed at 500 mg/m2 (n = 283)or docetaxel at 75 mg/m2 (n = 288)once every 21 days. There were nearlyoverlapping response rates, timesto progression, and median survivaltimes (Table 4). However, on thepemetrexed arm, there was significantlyless grade 3/4 neutropenia,febrile neutropenia, hospitalizationfor febrile neutropenia, hospitalizationfor adverse events, and alopecia,but significantly more transient elevationof alanine aminotransferaselevels.Pemetrexed Plus CisplatinWithout Vitamin Supplementation
In a phase I trial by Thodtmann etal, pemetrexed at 500 mg/m2 and cisplatinat 75 mg/m2 were tolerated ona schedule of once every 21 days.[43]It was conducted without vitamin supplementationbut was premedicatedwith dexamethesone incidental to premedicationof cisplatin. Responseswere seen in patients with NSCLC,mesothelioma, colorectal cancer, andhead and neck cancers. Neutropeniawas the dose-limiting toxicity. Pemetrexedand cisplatin had no observedpharmacokinetic interaction.Two phase II studies in chemonaiveNSCLC were conducted usingpemetrexed at 500 mg/m2 plus cisplatinat 75 mg/m2 without vitaminpremedication but with dexamethasoneprophylaxis. Shepherd et al accrued31 patients, with treatmentresulting in a 46% response rate, amedian survival of 8.9 months, and a1-year survival rate of 49%.[36] Hematologictoxicities (grade 3/4) wereneutropenia in 35% of patients, febrileneutropenia in 3%, anemia in19%, and thrombocytopenia in 3%.Nonhematologic toxicities (grade3/4) were fatigue in 26%, nausea in3%, vomiting in 3%, diarrhea in 10%,and stomatitis in 3%.The response rate was 39% with amedian time to progression of 6.3months and median overall survivalof 10.9 months in the 36-patient studyby Manegold et al.[37] Hematologictoxicities (grade 3/4) were neutropeniain 59% of patients, anemia in 14%,and thrombocytopenia in 17%. Grade3/4 nonhematologic toxicities werefatigue in 6% of patients, nausea/vomitingin 6%, diarrhea in 3%, and grade3 bilirubin and AST level elevations in 3% each. The Shepherd and Manegoldstudies showed activity comparableto that of the most activeregimens available.
Pemetrexed Plus CarboplatinWith Vitamin Supplementation
Two phase II studies of pemetrexedplus carboplatin completed accrualwith preliminary data reported: oneby Zinner et al[38] and the other byScagliotti et al.[39] Encouraging activityseen in the Manegold and Shepherd studies, the prevalence ofcarboplatin-based therapy, togetherwith preliminary data showing bettertolerability of pemetrexed in lung cancerwith vitamin use without loss ofefficacy, encouraged an updated lookat the efficacy and toxicity of pemetrexed/platinum doublets in first-lineadvanced NSLC. Patients were treatedwith pemetrexed at 500 mg/m2 andcarboplatin to an area under the concentration-time curve (AUC) of 6,with vitamin and dexamethasone prophylaxisin both studies. This regimenwas based on data obtained fromthe phase I mesothelioma trial (novitamin prophylaxis) by Hughes etal.[44]
In the study by Zinner et al, 50patients were accrued with a responserate of 28.8% (49 patients evaluablefor response). The median time to progressionwas 4.8 months, and with aminimum follow-up of 11 months,the median overall survival time isestimated at 13.5 months and the 1-year survival rate is estimated at55.8%. Patients could elect to receive> 6 cycles if they had no disease progression,although at the start of thestudy the intended number was 6 cycles.The median number of chemotherapycycles administered was 6 (range: 1 to > 15 cycles). Fifteen patients(30%) received ≥ 8 cycles (8cycles, n = 6, 10 cycles, n = 2; 12cycles, n = 4; 14 cycles, n = 2; 15cycles, n = 1). Greater than 277 cycleswere administered.Nonhematologic toxicity of grade≥ 3 was experienced by three patients(6%); grade 3 diarrhea, neutropenicinfection, and grade 3 fatigue all firstoccurred during the first six courses.Five patients had deep-vein thrombosesor pulmonary emboli. Giventhe low rate of clotting in the multipleother single-agent and doublet pemetrexedstudies, these are presumed notto be caused by this regimen. Theabsence of alopecia or neurosensorytoxicities of grade > 1 is notable. Fourpatients had grade 4 hematologic toxicities(grade 3 toxicities not yet tabulated)first occurring during the first 6courses, including one with neutropeniaand two with anemia.In this randomized phase II studyin previously untreated patients withadvanced NSCLC reported by Scagliottiet al,[39] patients were treatedwith either pemetrexed plus carboplatinexactly as in the study of Zinneret al[38] or pemetrexed at 500 mg/m2plus oxaliplatin (Eloxatin) at 120 mg/m2 on day 1 of each 21-day cycle. Of 80 patients, 39 patients were randomizedto pemetrexed/carboplatin and 41to pemetrexed/oxaliplatin. The responserate, median time to progression,and estimated median overallsurvival on the pemetrexed/carboplatinarm were 31.6%, 4.5 months, and9.9 months respectively. On the pemetrexed/oxaliplatin arm, response rate,median time to progression, and estimatedmedian overall survival were26.8%, 4.9 months, and 9.3 months,respectively.Patients treated with pemetrexed/carboplatin had grade 3/4 hematologictoxicities including neutropenia(26%), thrombocytopenia (18%), andanemia (8%). Grade 3/4 nonhematologictoxicities were fatigue (8%) andstomatitis (3%). On the pemetrexed/oxaliplatin arm grade 3/4 hematologictoxicities were neutropenia (7%)thrombocytopenia (2%), and anemia(2%) and grade 3/4 nonhematologictoxicities were vomiting (7%), neuropathy(2%), diarrhea (2%), and hypersensitivityreactions (2%).Both these studies showed activitycomparable to standard platinum doubletsbut with impressive tolerability,especially with pemetrexed plus carboplatin.The longer-than-expectedsurvival seen in the study by Zinner et al may be accounted for by the largenumber of patients receiving at leastsecond-line therapy upon progressing(76%), as compared to historical controls.The large number of cycles in15 of the patients, at the very least,demonstrate impressive tolerability,though it is not clear from this studywhether it offered benefit. Randomizedtrials are indicated.Pemetrexed CombinationsWith Nonplatinum Agents
There have been three phase IIstudies of pemetrexed-based nonplatinumregimens used to treat NSCLCreported. Two involved pemetrexed/gemcitabine and the other pemetrexed/vinorelbine. In one study by Monneratet al, gemcitabine at 1,250 mg/m2was given on days 1 and 8 with pemetrexedat 500 mg/m2 following gemcitabineon day 8 of a 21-daycycle.[40] The administration of vitaminswas introduced after 3 patientshad completed treatment and while10 patients were undergoing treatment,and all 60 patients received dexamethasone.The response, median progressionfreesurvival, median overall survival,and 1-year survival rate were 17%,4.9 months, 11.3 months, and 44%,respectively. Grade 3/4 hematologictoxicities were neutropenia (63%),anemia (12%), and thrombocytopenia(5%). Febrile neutropenia occurredin 15% of patients. Grade 3 fatigueoccurred in 23% of patients. Therewas an impressive survival, but a lower-than-expected response rate thatmay be ascribed to dose reductionsand delays.To further explore this, a phase IIby Adjei et al was done looking atalternative schedules of gemcitabineat 1,250 mg/m2 and pemetrexed at500 mg/m2.[41] None of the followingthree schedules were the same asin the Monnerat study[40]: (A) pemetrexedfollowed by gemcitabine onday 1, gemcitabine on day 8; (B) gemcitabinefollowed by pemetrexed onday 1, gemcitabine on day 8; and (C)gemcitabine on day 1, pemetrexed followedby gemcitabine on day 8 (whichwas the schedule used in the earlierstudy). Schedule B was stopped earlybecause of low response at interim analysis. Patients on arm A had lesssevere toxicity than patients on arm Cwith ≥ grade 3 events: 86% vs 93%(grade 4 events: 40% vs 50%). Responseon arm A was 29%, therebymeeting the protocol-defined efficacycriteria, whereas arm C with partialresponse of 17% did not. Theauthors recommend schedule A (pemetrexedfollowed by gemcitabine onday 1 and gemcitabine on day 8) forfurther study.The second nonplatinum regimenused pemetrexed at 500 mg/m2 onday 1 and vinorelbine at 30 mg/m2 ondays 1 and 8 of a 21-day cycle withvitamin supplementation.[42] Amongthe 34 patients, response was 35%.Survival data were not reported. Toxicitiesincluded grade 4 neutropenia(44%), febrile neutropenia (11%), andone treatment-related death. Twentyonepercent of patients had grade 3/4fatigue. This regimen has an activitysimilar to standard platinum doublets,but it may be more toxic than thecarboplatin-containing regimen.DiscussionPemetrexed is approved by theFDA for use in combination with cisplatinin the treatment of malignantpleural mesothelioma; it was the firstagent to show improved survival inthis disease.[25] In second-lineNSCLC it was equally effective, buthad superior tolerability compared todocetaxel in the largest second-lineNSCLC phase III study yet reported.[26] In addition, it showed activitysimilar to historical controls formedby third-generation agents in multiplephase II studies of pemetrexed aloneor in combination. The phase III mesotheliomatrial showed that the additionof vitamin prophylaxis improvedthe tolerability without evident negativeeffects on activity.The combination of pemetrexedwith carboplatin, cisplatin, or oxaliplatinis effective and safe, with activitycomparable to any of thestandard third-generation platinumdoublet regimens. Phase II studies ofpemetrexed/carboplatin with dexamethasoneplus vitamins, in particular,showed powerful efficacy combinedwith a low incidence of grade 3/4 hematologic and nonhematologic toxicities.The excellent tolerability isapparent when compared to historicalcontrols (Table 1). This suggests thatthis regimen may offer important improvementsover standard regimens,a conclusion that would be consistentwith the phase III single-agent second-line experience.This is reinforced by the observationthat there was minimal toxicityof pemetrexed/carboplatin in multiplecourses beyond 6 seen in the studyby Zinner et al[38]; however, whethercourses beyond 4 or 6 offer anyanticancer benefit will require otherstudies to determine. Pemetrexed isalso a convenient regimen, requiringadministration once every 21 days.Building on an earlier study lookingat single-agent pemetrexed with folicacid,[33] there are phase I studies underway evaluating pemetrexed at dosesof > 500 mg/m2 in combinationwith both folate and vitamin B12, giventhe increased tolerability withoutdecrease in efficacy observed withsuch prophylaxis in other studies.Combinations of nonplatinumagents with gemcitabine and vinorelbinein phase II studies have shownactivity similar to that of standard platinum-containing doublets but withoutimprovement in the toxicityprofile. A recent presentation of resultsfrom a study of three dosingschemes showed best tolerability andactivity with pemetrexed followed bygemcitabine on day 1, gemcitabineon day 8, a schedule that was bothmore active and less toxic than thepemetrexed plus gemcitabine scheduleevaluated in an earlier study. Furtherstudy will be required to clarifythe activity and tolerability.Given the efficacy and minimalsevere and long-lasting toxicities (notablyneurotoxicity) of pemetrexed incombination with platinum agents,these regimens should be studied aspart of multimodality therapy in earlier-stage disease. In the case of surgery,such considerations are ofincreased relevance given data presentedat the annual meetings of theAmerican Society of Clinical Oncologyin 2003 and 2004 showing clearand substantial reduction in the hazardratio in those receiving standard platinum-containing doublets as adjuvanttherapy.[47-49] Indeed, givenits excellent tolerability, pemetrexedregimens may enable better complianceas postsurgical adjuvant therapy,a condition critical to providing abest chance at eradicating microscopicpostsurgical disease.Pemetrexed is being studied incombination with radiation becausethere is preclinical evidence that it isa radiosensitizer. Full therapeutic dosesof pemetrexed can be combinedwith therapeutic doses of chest radiationtherapy as shown in a phase Idose-escalation study presented byVokes et al.[50] Pemetrexed plus carboplatinis being dose-escalated concurrentlywith chest radiation inanother study that is presently underway. It is also an attractive candidatefor combination with other new agentsgiven excellent efficacy and tolerability.Thus, in addition to combiningit with other modalities, pemetrexedbasedtreatment should be consideredas a chemotherapy platform uponwhich to add novel therapy. Randomizedtrials comparing pemetrexed doubletsto other doublets are alsoindicated.
Disclosures:
Dr. Zinner and Dr.Herbst have participated in speakers’ bureausand have been members of advisory boards forEli Lilly.
References:
1.
Jemal A, Tiwari RC, Murray T, et al: Cancerstatistics, 2004. CA Cancer J Clin 54:8-29,2004.
2.
Surveillance, Epidemiology, and EndResults Program, 1973-1999. Bethesda, Md:Division of Cancer Control and PopulationSciences, National Cancer Institute, 2002.
3.
Non-small cell lung cancer collaborativegroup: chemotherapy in non-small cell lungcancer: A meta-analysis using updated data onindividual patients from 52 randomized clinicaltrials. Br Med J 311:899-909, 1995.
4.
Roszkowski K, Pluzanska A, KrzakowskiM, et al. A multicenter, randomized, phase IIIstudy of docetaxel plus best supportive careversus best supportive care in chemotherapynaivepatients with metastatic or non-resectablelocalized non-small cell lung cancer (NSCLC).Lung Cancer 27:145-157, 2000.
5.
Ranson M, Davidson N, Nicolson M, etal: Randomized trial of paclitaxel plus supportivecare versus supportive care for patientswith advanced non-small-cell lung cancer.J Natl Cancer Inst 92:1074-1780, 2000.
6.
Gridelli C: The ELVIS trial: A phase IIIstudy of single-agent vinorelbine as first-linetreatment in elderly patients with advancednon-small-cell lung cancer-Elderly Lung CancerVinorelbine Italian Study. Oncologist6(suppl 1):4-7, 2001.
7.
Anderson H, Hopwood P, Stephens RJ, etal. Gemcitabine plus best supportive care (BSC)vs BSC in inoperable non-small cell lung cancer-a randomized trial with quality of life asthe primary outcome. UK NSCLC GemcitabineGroup. Br J Cancer 83:447-453, 2000.
8.
Negoro S, Masuda N, Takada Y, et al.Randomised phase III trial of irinotecan combinedwith cisplatin for advanced non-smallcelllung cancer. Br J Cancer 88:335-341, 2003.
9.
Sandler AB, Nemunaitis J, Denham C, etal. Phase III trial of gemcitabine plus cisplatinversus cisplatin alone in patients with locallyadvanced or metastatic non-small cell lung cancer.J Clin Oncol 18:122-130, 2000.
10.
Wozniak AJ, Crowley JJ, Balcerzak SP,et al. Randomized trial comparing cisplatinwith cisplatin plus vinorelbine in the treatmentof advanced non-small-cell lung cancer: ASouthwest Oncology Group study. J Clin Oncol16:2459-2465, 1998.
11.
Le Chevalier T, Brisgand D, Soria JC, etal: Long-term analysis of survival in the Europeanrandomized trial comparing vinorelbine/cisplatin to vindesine/cisplatin and vinorelbinealone in advanced non-small cell lung cancer.Oncologist 6(suppl 1):8-11, 2001.
12.
Giaccone G, Splinter TA, Debruyne C,et al: Randomized study of paclitaxel-cisplatinversus cisplatin-teniposide in patients with advancednon-small-cell lung cancer. The EuropeanOrganization for Research and Treatmentof Cancer Lung Cancer Cooperative Group. JClin Oncol16:2133-2141, 1998.
13.
Kubota K, Watanabe K, Kunitoh H, etal. Phase III randomized trial of docetaxel pluscisplatin versus vindesine plus cisplatin in patientswith stage IV non-small-cell lung cancer:The Japanese Taxotere Lung Study Group.J Clin Oncol 22:254-261, 2004.
14.
Smit EF, Van Meerbeeck JP, Lianes P, etal: Three-arm randomized study of twocisplatin- based regimens and paclitaxel plusgemcitabine in advanced non-small-cell lungcancer: A phase III trial of the European Organizationfor Research and Treatment of CancerLung Cancer Group-EORTC 08975. JClin Oncol 21:3909-3917, 2003.
15.
Scagliotti GV, De Marinis F, Rinaldi M,et al: Phase III randomized trial comparingthree platinum-based doublets in advancednon-small-cell lung cancer. J Clin Oncol20:4285-4291, 2002.
16.
Fossella F, Pereira JR, von Pawel J, etal: Randomized, multicenter, phase III studyof docetaxel plus platinum combinations versusvinorelbine plus cisplatin for advancednonsmall-cell lung cancer: The TAX326 studygroup. J Clin Oncol 21:3016-3024, 2003.
17.
Kelly K, Crowley J, Bunn PA Jr, et al:Randomized phase III trial of paclitaxelplus.carboplatin versus vinorelbine pluscisplatin in the treatment of patients with advancednonâsmall-cell lung cancer: A SouthwestOncology Group trial. J Clin Oncol19:3210-3218, 2001.
18.
Schiller JH, Harrington D, Belani CP, etal: Comparison of four chemotherapy regimensfor advanced non-small-cell lung cancer. NEngl J Med 346:92-98, 2002
19.
Fossella FV, DeVore R, Kerr RN, et al:Randomized phase III trial of docetaxel versusvinorelbine or ifosfamide in patients with advancednon-small-cell lung cancer previouslytreated with platinum-containing chemotherapyregimens. The TAX 320 Non-Small Cell LungCancer Study Group. J Clin Oncol 18:2354-2362, 2000.
20.
Shepherd FA, Dancey J, Ramlau R, etal. Prospective randomized trial of docetaxelversus best supportive care in patients with nonsmall-cell lung cancer previously treated withplatinum-based chemotherapy. J Clin Oncol18:2095-2103, 2000.
21.
Fukuoka M, Yano S, Giaccone G, et al:Multi-Institutional randomized phase II trial ofgefitinib for previously treated patients withadvanced nonâsmall-cell lung cancer. J ClinOncol 21:2237-2246, 2003.
22.
Kris MG, Natale RB, Herbst RS, et al:Efficacy of gefitinib, an inhibitor of epidermalgrowth factor receptor tyrosine kinase, in symptomaticpatients with non-small cell lung cancer:A randomized trial. JAMA 290:2149-2158,2003.
23.
Zinner RG, Herbst RS,Pemetrexed in thetreatment of advanced non-small-cell lung cancer:a review of the clinical data. Clin LungCancer April:5 (suppl 2):S67-S74, 2004.
24.
Adjei AA: Pemetrexed (Alimta): A novelmultitargeted antifolate agent. Expert RevAnticancerTher 3:145-156, 2003.
25.
Vogelzang NJ, Rusthoven JJ,Symanowski J, et al: Phase III study ofpemetrexed in combination with cisplatin versuscisplatin alone in patients with malignantpleural mesothelioma. J Clin Oncol 21:2636-2644, 2003.
26.
Hanna NH, Shepherd FA, Rosell R, etal: A phase III study of pemetrexed vs docetaxelin patients with recurrent non-small cell lungcancer (NSCLC) who were previously treatedwith chemotherapy (abstract 2503). Proc AmSoc Clin Oncol 22:622, 2003.
27.
Shih C, Chen VJ, Gossett LS, et al:LY231514, a pyrrolo[2,3-d]pyrimidine-basedantifolate that inhibits multiple folate-requiringenzymes. Cancer Res 57:1116-1123, 1997.
28.
Niyikiza C, Baker SD, Seitz DE, et al:Homocysteine and methylmalonic acid: Markersto predict and avoid toxicity frompemetrexed therapy. Mol Cancer Ther 1:545-552, 2002.
29.
Bunn P, Paoletti P, Niyikiza C, et al: VitaminB12 and folate reduce toxicity of Alimta(pemetrexed disodium, LY231514, MTA), anovel antifolate/antimetabolite (abstract 300).Proc Am Soc Oncol 20:76a, 2001.
30.
McDonald AC, Vasey PA, Adams L, etal: A phase I and pharmacokinetic study ofLY231514, the multitargeted antifolate. ClinCancer Res 4:605-610, 1998.
31.
Rinaldi DA, Burris HA, Dorr FA, et al:Initial phase I evaluation of the novelthymidylate synthase inhibitor, LY231514, usingthe modified continual reassessmentmethod for dose escalation. J Clin Oncol13:2842-2850, 1995.
32.
Rinaldi DA, Kuhn JG, Burris HA, et al:A phase I evaluation of the multitargetedantifolate (MTA, LY231514), administeredevery 21 days, utilizing the modified continualreassessment method for dose escalation. CancerChemother Pharmacol 44:372-380, 1999.
33.
Hammond L, Baker SD, Villalona-Calero SG, et al: A phase I and pharmacokineticstudy of the multitargeted antifol (MTA)LY231514 with folic acid (abstract). Ann Oncol9:620, 1998.
34.
Rusthoven JJ, Eisenhauer E, Butts C, etal: Multitargeted antifolate LY231514 as firstline chemotherapy for patients with advancednon-small-cell lung cancer: a phase II study. JClin Oncol 17:1194-1199, 1999.
35.
Clarke SJ, Abratt R, Goedhals L, et al:Phase II trial of pemetrexed disodium(ALIMTA, LY231514) in chemotherapy-naivepatients with advanced non-small-cell lungcancer. Ann Oncol 13:737-741, 2002.
36.
Shepherd FA, Dancey J, Arnold A, et al.Phase II study of pemetrexed disodium, amultitargeted antifolate, and cisplatin as firstlinetherapy in patients with advanced nonsmallcell lung carcinoma. Cancer 92:595-600, 2001.
37.
Manegold C, Gatzemeier U, von PawelJ, et al: Front-line treatment of advancednonsmall-cell lung cancer with MTA(LY231514, pemetrexed disodium, Alimta) andcisplatin: A multicenter phase II trial. AnnOncol 11:435-440, 2000.
38.
Zinner R, Obasaju CK, Fossella FV, etal: Alimta plus carboplatin (AC) in patients (pts)with advanced non-small-cell lung cancer(NSCLC): A phase II trial (abstract 7074). ProcAm Soc Clin Oncol 23:631, 2004.
39.
Scagliotti G, Kortsik C, Castellano D, etal: Phase II randomized study of pemetrexed +carboplatin or oxaliplatin, as front-line chemotherapyin patients with locally advanced ormetastatic non-small cell lung cancer (abstract2513). Proc Am Soc Clin Oncol 22:625, 2003.
40.
Monnerat C, LeChevalier T, Novello S,et al: Clinical results from a phase II trial ofpemetrexed + gemcitabine in advanced nonsmallcell lung cancer (abstract 483PD). AnnOncol 13(suppl 5):132, 2002.
41.
Adjei A, Nair S, Reuter N, et al:Pemetrexed (Pem)/gemcitabine (Gem) as frontlinetherapy for advanced NSCLC: A randomized,phase II trial of three schedules (abstract7070). Proc Am Soc Clin Oncol 23:630, 2004.
42.
Clarke S, Underhill C, White S, et al:Phase II study of pemetrexed and vinorelbinein patients with locally advanced or metastaticnon-small cell lung cancer (abstract #546P).Ann Oncol 13(suppl 5):149, 2002.
43.
Smit EF, Mattson K, von Pawel J, et al:Alimta (pemetrexed disodium) as second-linetreatment of non-small-cell lung cancer: Aphase II study. Ann Oncol 14:455-460, 2003.
44.
Thodtmann R, Depenbrock H, DumezH, et al. Clinical and pharmacokinetic phase Istudy of multitargeted antifolate (LY231514)in combination with cisplatin J Clin Oncol17:3009-3016, 1999.
45.
Hughes A, Calvert P, Azzabi A, et al.Phase I clinical and pharmacokinetic study ofpemetrexed and carboplatin in patients withmalignant pleural mesothelioma. J Clin Oncol20:3533-3544, 2002.
46.
Adjei AA, Erlichman C, Sloan JA, et al:Phase I and pharmacologic study of sequencesof gemcitabine and the multitargeted antifolateagent in patients with advanced solid tumors.J Clin Oncol 18:1748-1757, 2000.
47.
The International Adjuvant Lung CancerTrial Collaborative Group. Cisplatin-basedadjuvant chemotherapy in patients with completelyresected non-small-cell lung cancer. NEngl J Med 350:351-360, 2004.
48.
Strauss GM, Herndon J, Maddaus MA,et al: Randomized clinical trial of adjuvantchemotherapy with paclitaxel and carboplatinfollowing resection in stage IB non-small celllung cancer (NSCLC): Report of Cancer andLeukemia Group B (CALGB) Protocol 9633(abstract 7019). Proc Am Soc Clin Oncol 23:2004.
49.
Winton TL, Livingston R, Johnson D, etal: A prospective randomised trial of adjuvantvinorelbine (VIN) and cisplatin (CIS) in completelyresected stage 1B and II non small celllung cancer (NSCLC) Intergroup JBR.10 (abstract7018). Proc Am Soc Clin Oncol 23:2004.
50.
Vokes E, Szeto L, Mauer A, et al: A phaseI trial of pemetrexed + chest radiotherapy inpatients with advanced or metastatic non-smallcelllung cancer or esophageal cancer (abstract#O-89). Lung Cancer 41(suppl 2):S89, 2003.