Chemotherapy-Induced Cardiomyopathy: Clinical Scenarios and Challenges
We will discuss the deleterious cardiovascular effects of anthracyclines and HER2-targeted agents in a case-based format, as well as specific approaches to prevention and treatment of associated cardiotoxicity.
Oncology (Williston Park). 29(10):730-732, 786.

Table. Risk Factors for Anthracycline-Induced Cardiomyopathy
Introduction
Anthracyclines and human epidermal growth factor receptor 2 (HER2)-targeted agents play a key role in improving disease-free survival in breast cancer, sarcomas, and some hematologic malignancies. However, the cardiotoxicity of these agents can be clinically challenging and may adversely affect prognosis. We will discuss these commonly used chemotherapeutic agents and their deleterious cardiovascular effects in a case-based format, as well as specific approaches to prevention and treatment of associated cardiotoxicity.
#1 Clinical Vignette
A 29-year-old man with no known cardiac history was diagnosed with mesenchymal chondrosarcoma in the spine and underwent surgical resection, anthracycline-based chemotherapy, and adjuvant radiation. Over the next 10 years, he had three recurrences of chondrosarcoma, two in the lung and one in the abdomen, which were treated with surgical resection with or without anthracycline-based chemotherapy. Surveillance echocardiograms done before, during, and after anthracycline therapy showed normal left ventricular ejection fraction (LVEF). At age 41, he was cancer-free and asymptomatic. Since he had received a cumulative dose of 520 mg/m2 of doxorubicin, an echocardiogram was obtained, which showed an LVEF of 42%. A stress test was negative for ischemia. He was diagnosed with late anthracycline-induced cardiomyopathy and was optimized on medical therapy, including beta blockers and angiotensin-converting enzyme (ACE) inhibitors. At 5-month follow-up, his LVEF had improved to 52%; he remains asymptomatic.
Who is at risk for anthracycline cardiotoxicity?
Cardiotoxicity is a dose-limiting side effect of the anthracyclines doxorubicin, daunorubicin, epirubicin, and mitoxantrone. Acute cardiotoxicity is uncommon, usually occurring within 1 week after treatment completion, and can present with cardiomyopathy, arrhythmias, myocardial infarction, and myopericarditis/pericarditis syndromes.[1] With early treatment and discontinuation of the anthracycline, it is generally reversible. In some cases, acute cardiotoxicity may, in fact, be a form of stress-induced cardiomyopathy.
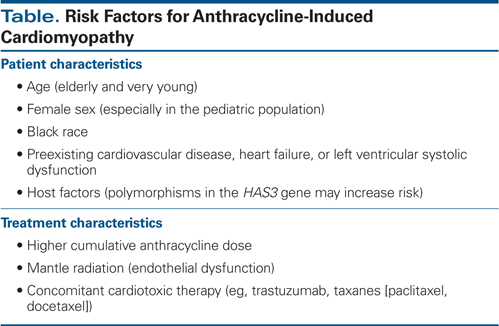
Anthracycline-induced cardiomyopathy manifesting as subacute and chronic cardiotoxicity is more common.[2] Congestive heart failure as a result of systolic and/or diastolic dysfunction is dose-dependent. Although historically the emphasis has been on systolic dysfunction, heart failure due to diastolic dysfunction is now being increasingly identified. A recent study showed a high incidence of diastolic dysfunction in patients after treatment with an anthracycline with or without trastuzumab.[3] Age and body mass index were independent predictors of diastolic dysfunction in this study.[3] The most important risk factor for cardiomyopathy is the cumulative dose of anthracyclines. Although the risk is low below 300 mg/m2, it exponentially increases with dose and is estimated at 26% for 550 mg/m2 and 48% for 700 mg/m2.[4] Susceptibility to cardiomyopathy is increased by various other host and treatment factors (Table).[5,6]
Topoisomerase (Top)2-alpha (overexpressed in tumors) is the cellular target for the drug’s anticancer effect. DNA damage via Top2-beta (expressed in adult cardiomyocytes) leading to cardiomyocyte death has recently been implicated as a major mechanism of anthracycline-related cardiomyopathy.[7]
Monitoring anthracycline cardiotoxicity
Pretreatment assessment and subsequent monitoring help with early detection of cardiac dysfunction. Two-dimensional (2D) echocardiography is the approach most frequently used to monitor LVEF, due to its availability, cost-effectiveness, and lack of radiation. Limitations include inadequate acoustic windows and variability. Three-dimensional (3D) echocardiography and cardiac magnetic resonance (CMR) imaging have superior accuracy, especially relevant to screening for borderline left ventricular (LV) dysfunction.[8] Abnormalities in global longitudinal strain are a sign of subclinical myocardial dysfunction caused by anthracycline therapy, occur prior to change in 2D LVEF, and are more reproducible measurements than 2D LVEF. Importantly, these abnormalities predict the development of subsequent cardiotoxicity.[9]
Cardiac biomarkers, including troponin I and brain natriuretic peptide, are markers of early cardiac damage. In some studies, troponin I levels have been shown to predict future risk of cardiomyopathy; however, further validation is needed.[10]
There is a lack of evidence-based guidelines for monitoring patients during and after anthracycline therapy. However, expert opinion/consensus documents can serve as valuable resources.
Our clinical approach is similar to that recommended in the recently published expert consensus report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. We perform baseline evaluation of LVEF with 2D echocardiography (or 3D echocardiography, when available) prior to anthracycline-based chemotherapy. When feasible, global longitudinal strain can be additionally obtained. The cost-benefit of pre-therapy LV function assessment has been questioned, however. We recommend follow-up at completion of therapy and 6 months later for doses less than 240 mg/m2. Once this dose is exceeded, repeat assessment is recommended before each additional 50 mg/m2.[11] If any of the LV function parameters are abnormal or show a significant change from baseline, cardiology consultation should be obtained for consideration of medical therapy, risk-benefit discussion, and closer monitoring. For high-risk patients (eg, the elderly or patients with preexisting cardiac disease), we often employ a closer clinical and imaging surveillance schedule.[12]
Treating and preventing anthracycline cardiotoxicity
Historically, subacute and chronic forms of anthracycline-induced cardiomyopathy have been considered irreversible, and as such, labeled type I chemotherapy-related cardiac dysfunction (CRCD). However, recent studies have shown that with careful surveillance and early treatment, anthracycline-induced cardiomyopathy can be successfully treated.[13,14] Although there is no specific treatment for anthracycline-induced cardiotoxicity, the experience is that patients respond well to guideline-concordant medical therapy. Beta blockers and ACE inhibitors are the cornerstone of medical management. Appropriate patients should also be referred for advanced heart failure and device therapies when indicated.
Strategies to limit or prevent anthracycline-induced cardiomyopathy include limiting anthracycline dosage, use of doxorubicin derivatives, longer infusion durations, and concomitant use of the cardioprotective agent dexrazoxane.[15] Animal studies have suggested that pretreatment with statins may be preventive.[16] The benefit of preventive beta blockers and ACE inhibitors has recently been investigated, with promising results[17]; however, larger confirmatory studies are needed prior to using these agents prophylactically.
#2 Clinical Vignette
A 48-year-old woman with no cardiac history was diagnosed with breast cancer (estrogen receptor– and HER2-positive) metastatic to the brain. She was treated with left mastectomy as well as stereotactic radiosurgery of brain metastases, continuing on a trastuzumab-based regimen. Clinical cardiovascular status and surveillance parameters remained normal until 2 years into therapy, at which time she was admitted with acute systolic heart failure and an echocardiographic 2D LVEF of 33%. She was diuresed and optimized on heart failure medications, including beta blockers and ACE inhibitors. Evaluation showed no evidence of ischemic heart disease, and she was diagnosed with trastuzumab-induced cardiomyopathy. Trastuzumab was discontinued and LVEF slowly recovered to normal levels over the next 4 weeks. She was retreated with trastuzumab while continuing her heart failure therapies and tolerated it well without recurrence of LV systolic dysfunction or clinical heart failure.
Who is at risk for trastuzumab cardiotoxicity?
HERs are transmembrane tyrosine kinase receptors that normally regulate vital cellular responses.[18] Approximately 20% to 30% of patients with breast cancer have the subtype characterized by HER2/neu gene amplification, which is associated with more aggressive disease.[19] Trastuzumab is a monoclonal antibody that targets the extracellular domain of the HER2/neu tyrosine kinase[20]; it substantially reduces the risk of recurrence and early death in women with HER2-positive breast cancer.[21,22]
Cardiotoxicity is an important side effect of trastuzumab. The mechanism of trastuzumab-related cardiotoxicity is thought to be blocking of the HER2 pathway and its downstream signaling in cardiomyocytes, which disrupts the protective role of HER2 signaling in cardiomyocyte growth, repair, and survival.[18,23,24] Animal studies have shown that disruption in signaling through HER2 in cardiomyocytes causes dilated cardiomyopathy[25,26] and mitochondrial dysfunction in cardiomyocytes.[27]
The incidence of cardiotoxicity (manifested as abnormal LVEF and congestive heart failure) ranges from 3% to 7% with trastuzumab monotherapy, is 13% with paclitaxel and trastuzumab, and is 27% with concomitant anthracycline and trastuzumab.[28] Up to 16% of patients in the pivotal trials receiving combined anthracycline and trastuzumab have New York Heart Association class III or IV heart failure,[28] and thus concomitant administration should be avoided. Preexisting cardiac disease, older age, diabetes mellitus, and prior cardiotoxic therapy lead to an increased incidence of cardiotoxicity.[29] The cardiotoxicity caused by trastuzumab appears to be different from that caused by anthracyclines in that it occurs as a result of myocyte dysfunction rather than myocyte death, is often reversible, and historically has been labeled type II CRCD. However, it is important to understand that reversibility can be variable depending on the time of detection and treatment, and significant overlap occurs between type I and type II CRCD.
Pertuzumab is an antibody against HER2 dimerization. Meta-analysis of LV dysfunction in clinical trials using mono vs dual HER2 blockade (pertuzumab and trastuzumab vs lapatinib and trastuzumab vs trastuzumab alone) showed similar cardiotoxicity between all three groups.[30]
Monitoring and treating trastuzumab cardiotoxicity
Our practice for surveillance of cardiotoxicity due to trastuzumab is to obtain a 2D LVEF (or 3D LVEF, where available) at baseline. Global longitudinal strain should be obtained if feasible. After starting therapy, these assessments should be repeated every 3 months until completion of therapy.[11] If baseline or monitoring parameters are abnormal and/or show a significant change from baseline, cardiology consultation should be obtained. Trastuzumab is usually held for at least 4 weeks if there is a ≥ 16% absolute drop in LVEF, or if LVEF falls below normal with a ≥ 10% absolute decrease from baseline. CMR imaging is helpful for LVEF confirmation if echocardiographic image quality is inadequate when therapy interruption is being considered. Trastuzumab-related cardiotoxicity is usually reversible and not dose-related.[31] It usually responds well to standard heart failure management, and treatment rechallenge with the drug should be attempted in select cases.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Bristow MR, Thompson PD, Martin RP, et al. Early anthracycline cardiotoxicity. Am J Med. 1978;65:823-32.
2. Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900-5.
3. Serrano JM, González I, Del Castillo S, et al. Diastolic dysfunction following anthracycline-based chemotherapy in breast cancer patients: incidence and predictors. Oncologist. 2015;20:864-72.
4. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-79.
5. Gupta D, Yadav N, Evans F, Steingart R. Chemotherapy-induced cardiomyopathy in the elderly. Curr Cardiovasc Risk Rep. 2014;8:1-8.
6. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710-7.
7. Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639-42.
8. Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:
2876-84.
9. Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751-68.
10. Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749-54.
11. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-39.
12. Gupta D, Verma S, Pun SC, Steingart RM. The changes in cardiac physiology with aging and the implications for the treating oncologist. J Geriatr Oncol. 2015;6:178-84.
13. Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981-8.
14. Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213-20.
15. Conway A, McCarthy AL, Lawrence P, Clark RA. The prevention, detection and management of cancer treatment-induced cardiotoxicity: a meta-review. BMC Cancer. 2015;15:366.
16. Riad A, Bien S, Westermann D, et al. Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res. 2009;69:695-9.
17. Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355-62.
18. Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol. 2008;44:831-54.
19. Hudziak RM, Schlessinger J, Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci USA. 1987;84:7159-63.
20. Albanell J, Bellmunt J, Molina R, et al. Node-negative breast cancers with p53(-)/HER2-neu(-) status may identify women with very good prognosis. Anticancer Res. 1996;16:1027-32.
21. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-92.
22. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-72.
23. Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332-44.
24. Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954-60.
25. Ozcelik C, Erdmann B, Pilz B, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:8880-5.
26. Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459-65.
27. Grazette LP, Boecker W, Matsui T, et al. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2231-8.
28. Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-21.
29. Serrano C, Cortes J, De Mattos-Arruda L, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol. 2012;23:897-902.
30. Valachis A, Nearchou A, Polyzos NP, Lind P. Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer. 2013;133:2245-52.
31. Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820-6.