Commentary (Estey): The Role of Hematopoietic Stem Cell Transplantation in Myelodysplastic Syndrome
Drs. Thompson and Luger’spaper provides a comprehensivesurvey of issues surroundinghematopoietic stem celltransplantation (HSCT) in myelodysplasticsyndrome (MDS). Whilefinding much of value in the paper, Istrongly disagree with the authors’opinion that “it is clear that youngpatients with [human leukocyte antigen(HLA)]–identical siblings. . .should undergo allogeneic HSCT assoon as possible.” This view wouldseem to rest on two premises: first,that allogeneic HSCT is, as the authorscontend, the only therapy“shown to alter the natural history ofMDS,” and second, that results withallogeneic HSCT are sufficiently“good” that the procedure can be regardedas a fixed, standard elementof medical practice.
Drs. Thompson and Luger's paper provides a comprehensive survey of issues surrounding hematopoietic stem cell transplantation (HSCT) in myelodysplastic syndrome (MDS). While finding much of value in the paper, I strongly disagree with the authors' opinion that "it is clear that young patients with [human leukocyte antigen (HLA)]-identical siblings. . . should undergo allogeneic HSCT as soon as possible." This view would seem to rest on two premises: first, that allogeneic HSCT is, as the authors contend, the only therapy "shown to alter the natural history of MDS," and second, that results with allogeneic HSCT are sufficiently "good" that the procedure can be regarded as a fixed, standard element of medical practice. Treatment Alternative
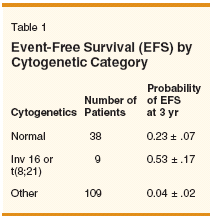
Regarding the purported uniqueness of transplant, in 1996 we published data suggesting that treatment of high-risk MDS (International Prognostic Scoring System [IPSS] categories intermediate-2 or high) with the same chemotherapy we gave acute myelogenous leukemia (AML) patients produced long-term results similar to those of allogeneic HSCT, at least as published by researchers at the Fred Hutchinson Center Research Center (and at least in young patients with normal cytogenetics).[1] Writing this commentary prompted an update. Between 1991 and 2000, we gave AML-type therapy to 156 patients under age 60 with MDS and > 5% blasts in either marrow or blood. Patients received a variety of regimens, linked by the use of cytarabine at a daily dose of 1 to 2 g/m2 for 4 to 5 days. The median age was 50, 115 had refractory anemia with excess blasts in transformation (RAEB-t), 38 had refractory anemia with excess blasts (RAEB), and 3 had chronic myelomonocytic leukemia. Overall, 57% were scored as IPSS high and 43% as IPSS intermediate-2. The probability of event-free survival was 0.12 ± .03 at 3 years and 0.11 ± .03 at 5 years. I quote the 3-year figure both because it is frequently used by Drs. Thompson and Luger, and because patients can be considered "potentially cured" once they have been in remission this long. In particular, as shown by the similarity between the 3- and 5-year event-free survival rates, the failure rate becomes very low after 3 years. More important than the overall results noted above are those within different cytogenetic categories (Table 1). Superior Outcome?
Hence, patients under age 60 with a normal karyotype, and the rare younger patient with MDS and inv(16) or t(8;21), can be cured with AML-type therapy as well as allogeneic HSCT. An obvious question is whether outcome is better with the former or with the latter. Because the proponents of AML-type therapy and of allogeneic HSCT each have such strongly held and conflicting opinions, attempts to answer this question by randomizing patients with an available donor between allogeneic HSCT and AML-type therapy are not feasible. Nor, as far as I know, have there been analyses intended to assess whether-after accounting for covariates such as age, duration of abnormal blood counts, or presence of FLT3 or CEBP-alpha mutations-the survival or event-free survival of patients with MDS and, for example, a normal karyotype is affected by use of allogeneic HSCT, rather than AML-type therapy.
There have, however, been reports of the results of such analyses in patients given allogeneic HSCT; these enable us to estimate outcome in different patient groups. For example, the International Bone Marrow Transplant Registry published outcomes of allogeneic HSCT in 452 patients with MDS, whose median age was 38, with 60% having RAEB or RAEB-t.[2] At the time of allogeneic HSCT, 38% of patients were in complete remission (CR). Patients under age 18 with < 5% marrow blasts had an 80% probability of long-term event-free survival, but relative risk increased 2.5-fold for patients above age 45 and, independently of age, 2.1-fold for patients with > 5% marrow blasts. Multiplying 2.5 by 2.1 and applying the product to the 80% baseline rate gives a long-term success rate of 15%, hardly different from the M. D. Anderson results with AML-type chemotherapy. Furthermore, in settings (eg, first-remission AML) in which covariate-adjusted comparisons have been drawn between allogeneic HSCT and AML-type therapy, outcome is dictated by the covariates-eg, cytogenetics, FLT3 status-rather than by the treatment received.[3] Impact of Patient Selection
Such analyses suffer greatly from our inability to know the extent to which patient selection influences the result. As an example, we recently designed a study calling for all patients over age 50 with AML in first CR to receive a reduced-intensity allogeneic HSCT if an HLA-compatible donor was available. Among 99 such patients, only 62 had the transplant consult that was a prerequisite to HLA-type potential donors, and only 11 of the 25 with donors had a transplant. Although these 11 had by far the longest relapse-free survival, they represent only 27% of the patients with donors (assuming the proportion of patients with a donor to be independent of whether a consult was obtained). Hence, the effect of allogeneic HSCT is very plausibly confounded by the effect of patient selection. There is no reason to think that the same phenomenon does not apply in the context of new chemotherapy regimens. Conclusions
On a more fundamental level, the relative merits of allogeneic HSCT and AML-type therapy are irrelevant. In particular, neither therapy is "good" in any commonly accepted use of the word. Hence, in my opinion, efforts should be made to dissuade the great majority of high-risk MDS patients from receiving either conventional allogeneic HSCT or conventional AML-type therapy. Rather, incentives should be in place to employ investigational treatments, whether or not they involve allogeneic HSCT.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Anderlini P, Pierce S, Kantarjian H, et al: Acute myeloid leukemia type therapy for myelodysplasia. J Clin Oncol 14:1404-1405, 1996.
2. Sierra J, Perez W, Rozman C, et al: Bone marrow transplantation from HLA-identical siblings as treatment or myelodysplasia. Blood 100:1997-2004, 2002.
3. Burnett AK, Goldstone AH, Stevens R, et al: Biologic characteristics determine the outcome of allo or auto BMT in AML CR1. Blood 86:614a, 1995.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.