Commentary (Marshall): The Horizon of Antiangiogenic Therapy for Colorectal Cancer
Recent advances in understandingthe cellular mechanismsthat determine tumor developmentand progression havespawned a plethora of the so-called“smart therapies”-targeting variousaspects of aberrant cell signaling.Some of the most promising of theseinclude agents targeting tumor angiogenesis,among them the vascularendothelial growth factor (VEGF)-specific humanized monoclonal antibodybevacizumab (Avastin), whichis the most advanced antiangiogenicagent in clinical development. Theinitial promise of this agent is nowsupported by proof-of-concept clinicaldata and is discussed in the comprehensivereview by Olszewski andcolleagues. But the question remains:How does bevacizumab achieve thisclinical benefit?
Recent advances in understanding the cellular mechanisms that determine tumor development and progression have spawned a plethora of the so-called "smart therapies"-targeting various aspects of aberrant cell signaling. Some of the most promising of these include agents targeting tumor angiogenesis, among them the vascular endothelial growth factor (VEGF)- specific humanized monoclonal antibody bevacizumab (Avastin), which is the most advanced antiangiogenic agent in clinical development. The initial promise of this agent is now supported by proof-of-concept clinical data and is discussed in the comprehensive review by Olszewski and colleagues. But the question remains: How does bevacizumab achieve this clinical benefit? Angiogenesis and the Angiogenic Switch
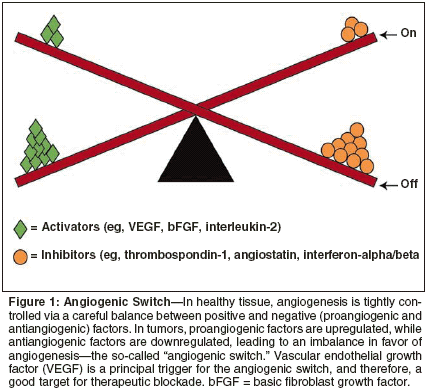
Angiogenesis is a normal physiologic process responsible for the growth of new blood vessels in wound healing, injury repair, the female reproductive cycle, and pregnancy. Defects in mechanisms controlling angiogenesis occur in various pathologic states, including cancer, whereby excessive tumor-driven neovascularization occurs. This dependence of tumor development and progression on the development of a nascent blood supply was first identified more than 30 years ago in the pioneering re search by Folkman and colleagues in the United States, and is now widely accepted.[1] Until it exceeds 1 to 2 mm in diameter, a tumor can sustain growth via simple diffusion of nutrients and oxygen across its surface. Once that critical surface-area-to-volume ratio has been breached, however, the tumor must initiate and establish its own blood supply, and this process is essential for further growth. Both physiologic and pathologic angiogenesis are regulated by a complex range of circulating growth factors originating from the tissue or tumor cells themselves, from surrounding tissues, or from infiltrating macrophages and fibroblasts.[ 2] These factors include mediators that are highly specific for endothelial cells, such as VEGF, and others with a broader spectrum of action, such as matrix metalloproteinases (MMPs).[3] These mediators are themselves regulated by an underlying genetic framework incorporating various proangiogenic oncogenes, eg, human epidermal growth factor receptor 2 (HER2), and tumor-suppressor genes such as p53. Prevascularized tumors may remain dormant for many years before conditions arise that are conducive for conversion to an angiogenic phenotype. This is elegantly illustrated by considerable evidence obtained at autopsy, in which a far higher frequency of "dormant" cancers is seen than is typically clinically diagnosed in respective patient populations.[4] Disruption of the delicate equilibrium between inhibitory and stimulatory factors, such as may arise from oncogene activation and/or suppressor gene dysfunction, triggers a conversion known as the "angiogenic switch" (Figure 1). Once the switch is in the "on" position, recruitment of mature host vasculature commences, and this is induced to begin budding new capillaries, which grow toward the tumor, infiltrating it.[5] Although such induction of new vessel formation and growth is the predominant mechanism for tumor-initiated angiogenesis ("sprouting"), research has shown that some tumors may initially sequester host vessels directly ("nonsprouting").[ 6] This vasculature subsequently regresses, resulting in tumor shrinkage that is consequently reversed by a conventional process of hypoxia-induced angiogenesis at the tumor edge. Biology of VEGF Signaling
Integral to both physiologic and pathologic angiogenesis is VEGF, also known as VEGF-A. This mediator belongs to the VEGF platelet-derived growth factor supergene family and exists as four genetically distinct isoforms.[ 3] Tumors produce large quantities of this growth factor in response to a multitude of drivers, including oncogenes and various gene product modulators. VEGF exerts a spectrum of actions almost exclusively affecting vascular endothelium, but also influencing specific components of cellular immunity.
These effects are initiated via highaffinity binding to the extracellular domains of two membrane-bound receptor tyrosine kinases, designated Flt-1 (VEGFR-1) and Flk-1/KDR (VEGFR-2), which are predominantly located on the surfaces of vascular endothelial cells.[7] Receptor binding of VEGF initiates cell signal transduction, although the two receptor subtypes have markedly different signaling properties. Flt-1 binds ligand with higher affinity than Flk-1/KDR, although the latter is believed to be the principal receptor responsible for initiating VEGF signaling; Flt-1 acts as a "decoy," regulating ligand availability to its coreceptor.[7,8] VEGF directly stimulates endothelial cell proliferation and promotes cell survival by inhibiting apoptosis,[ 9] an effect that is in part attributable to regulation of the phosphatidylinositol 3-kinase/Akt cell signaling pathway.[10] VEGF-mediated secretion and activation of enzymes, including MMPs, results in degradation of the extracellular matrix, permitting vascular remodeling.[11-13] Ulti- mately, VEGF modulates endothelial cell migration to the neovascularization site[14] and plays a fundamental role in recruiting and mobilizing bone marrow-derived endothelial progenitor cells.[15] Since tumors produce vast amounts of VEGF, a positive feedback loop is created whereby VEGF-driven angiogenesis enables tumor growth, which then allows for increased secretion of VEGF. This magnification may also be further amplified by VEGFmediated upregulation of target endothelial receptors.[16] Furthermore, tumors may themselves express VEGF receptors. Thus, VEGF has a dual role as both a paracrine mediator influencing vascular endothelial cell function, and an autocrine mediator promoting tumor cell growth and survival.[17-19] Characteristics of Tumor Vasculature
Tumor vasculature shows striking morphologic and functional differences compared with its normal, healthy counterpart. Tumor vessels are twist ed, excessively branched, of a variable diameter, and often dilated.[20] In addition, the integrity of their walls is severely compromised, with features including fenestration and open gaps, leading to significantly increased vessel permeability. Delivery of nutrients and oxygen to the tumor is compromised, leading to hypoxia and tissue necrosis, which themselves can further stimulate increased angiogenesis. Pathologic vasculature is also extremely friable and highly dependent on locally circulating growth factors for survival.[21] Targeting the VEGF Ligand in Cancer Therapy
The rationale for targeting VEGF is manifold. First, it is well established that tumors must develop their own blood supply in order to grow beyond a dormant state and that VEGF is a pivotal factor in this process, serving as a point of integration in a variety of upstream and downstream signals. Targeting only one VEGF receptor could be insufficient to prevent tumor angiogenesis, given the wide range of effects of VEGF and the mediation by different receptors in this process. VEGF promotes angiogenesis by acting directly on the endothelial cell, a genetically stable entity, and agents that act to inhibit VEGF may be less susceptible to the selection of mutations that confer resistance.[22] Next, there is extensive literature supporting the observation that expression of this factor is increased in most cancers examined thus far.[3] Studies in patients with breast, colorectal, or ovarian cancers have shown that tumor resection is accompanied by a dramatic decline in elevated circulating levels of VEGF, which tends to be reversed on recurrence, strongly supporting a causal association with disease progression.[23-25] Moreover, research has shown a link between angiogenesis and metastasis. VEGF-driven angiogenesis not only enables growth of the primary tumor, but provides a route for cancer cells to enter the host vascular system, and thence to spread to other organs.[26] Perhaps unsurprisingly, therefore, in several studies microvascular density of the primary tumor has been shown to correlate with clinical outcome, with increased microvascular density being a negative prognostic marker in most instances.[27] The intrinsic role of VEGF in the development of clinically manifest disease is also illustrated by the observation that VEGF is upregulated by genetic events similar to those known to be responsible for malignant transformation, such as loss of p53, and activation of HER2.[28] In addition, VEGF expression is upregulated by hypoxia, which is present in most tumors.[29] Importantly, therefore, the strategic and central role of VEGF in tumor angiogenesis, whereby it can both influence and be influenced by a host of other factors, renders it an excellent therapeutic target. Mode of Action of Bevacizumab
Bevacizumab is distinct from other agents targeting VEGF-driven tumor cell signaling for several important reasons. Unlike the situation in many other potential therapeutic approaches, the target for this monoclonal antibody-VEGF-is well characterized, and bevacizumab has high binding affinity to all four isoforms. Since VEGF plays such a pivotal role in tumor angiogenesis, this direct ligand-specific targeting by bevacizumab has the advantages of a more comprehensive blockade of signaling in the angiogenic pathway. It also permits inhibition not only of vascular endothelial cells and their precursors but also of VEGF function on certain immune cells, and thus may have a collateral benefit of improving immune surveillance of tumors.[26] The murine precursor antibody to bevacizumab, A4.6.1, effectively interrupts the VEGF-fueled angiogenic continuum, as exemplified by preclinical studies showing dose- and timedependent growth inhibition of human colon carcinoma xenografts in mice after single-agent administration.[30] This effect was accompanied by a marked reduction in the number and size of experimental liver metastases, which were also avascular. Early tri- als in cancer patients evaluating bevacizumab monotherapy showed promising results. In the randomized, phase II trial in patients with metastatic renal cell carcinoma, high-dose treatment with bevacizumab significantly prolonged time to disease progression, although overall survival was not affected.[31] Interestingly, recent research has confirmed that bevacizumab acts via antivascular effects in human cancer, normalizing vascular morphology and evening out erratic blood flow. A single infusion of bevacizumab in patients with rectal carcinoma decreased tumor perfusion, vascular volume, microvascular density, and interstitial fluid pressure, reduced the number of viable circulating endothelial and progenitor cells, and stabilized tumor vascular architecture.[32] These findings are in agreement with similar observations from preclinical studies in which A4.6.1 reduced tumor vascular permeability, with an associated decrease in interstitial pressure.[33,34] Vascular changes were correlated with inhibition of tumor growth. Reorganization of Tumor Vasculature by Bevacizumab
Preclinical and/or clinical evaluation of bevacizumab in combination with conventional cytotoxic agents,[35,36] with radiation,[34] with immunotherapy,[37] or with other biologic molecules[38] showed evidence of additive, and possibly synergistic, inhibitory effects on tumor growth. This enhancement appears to translate into a similar phenomenon in cancer patients, and bevacizumab activity with chemotherapy combinations are discussed by Olszewski and colleagues in this issue. Thereby exists a seeming paradox: Bevacizumab has an antivascular action on tumors, yet to be effective, chemotherapy must be delivered to the tumor via its own blood supply. Clinical experience suggests that these events can coexist, and even that they are compatible. The question is, how? Under the influence of VEGF, tumor- specific vasculature develops with several morphologic and functional abnormalities that detrimentally affect access to the tumor by conventional chemotoxic agents. For instance, the tumor vascular network is disorganized, with many "blind alleys" and anastomotic circuits, in which drug molecules may become diverted and routed away from target tumor tissue.[40] In addition, the increased vascular volume around the tumor dilutes the drug, meaning that a less effective concentration reaches tumor cells. To reach the tumor tissue, a drug must first enter the tumor via the vascular compartment, then cross the vessel wall, before moving through the interstitial compartment. Notably, drug penetration into tumor tissue is also significantly reduced, because most tumors exhibit substantially elevated interstitial fluid pressure as a result of increased vascular permeability and the absence of lymphatic drainage.[41,42] The resulting lack of any appreciable pressure difference across the tumor microvascular wall, therefore, impedes the movement of large molecules, including chemotherapeutic drugs, from the intravascular space to tumor tissue.[43] The VEGF signaling pathway is required for the maintenance of tumor vascular structure. Studies using animal models of human cancer have shown that even in established, vascularized cancer, inhibition of VEGF produces remarkable changes in vessel morphology. Moreover, VEGF blockade by bevacizumab is known to arrest and/or reverse many of the abnormalities of tumor vasculature.[3] This is entirely consistent with the theory that antiangiogenic enhancement of tumor cell killing by cytotoxic compounds is a function of "pruning" of abnormal vessels as a result of endothelial cell death.[40] Hence, coadministration of bevacizumab with chemotherapeutic agents probably works along the following lines (see Figure 2). The tumor secretes VEGF, which stimulates local neovascularization. Under continuous VEGF stimulation, tumor vas culature develops abnormally, and is maintained in a highly disorganized state. The extensive and dense network of vessels facilitates the spread of tumor cells into the host circulation, and underpins metastatic disease. Bevacizumab binds with VEGF, preventing it from binding to its target receptors, primarily located on endothelial cells. This ligand blockade inhibits formation of new vasculature and reduces microvascular density, concurrently normalizing dysfunctional vascular morphology and reducing the potential for metastasis.
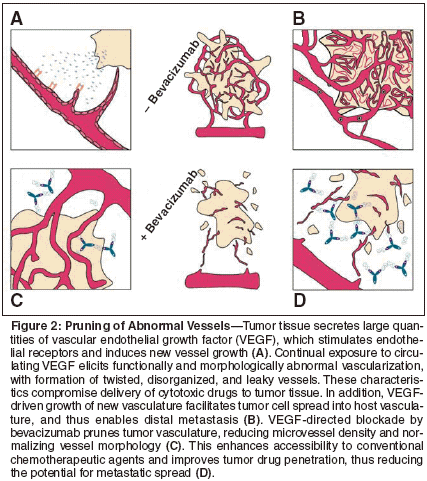
Bevacizumab activity is not appreciably affected by the raised interstitial fluid pressure around the tumor, since it does not need to reach or penetrate tumor tissue itself, but must simply bathe the immediate microvascular environment and host vascular space. However, once it has exerted its pruning effects on tumor vasculature, it creates improved conditions for chemotoxic drug accessibility and penetration. Diameter and tortuousity of vessels are reduced, as is local vascular volume, and excessive vascular permeability is ameliorated, thereby lowering interstitial fluid pressure. In consequence, there is less drug dilution, better delivery, and greatly improved penetration into tumor tissue, resulting in improved bioavailability. This hypothesis is supported by the landmark phase III clinical study in patients with metastatic colorectal cancer, in whom the addition of bevacizumab to fluorouracil-based chemotherapy significantly improved survival.[39] To confirm synergy, levels of cytotoxic agents in tumor tissue, with and without coexposure to bevacizumab, would need to be measured. Since this may be impractical in human subjects, animal models could be used. However, there is evidence from animal studies that the precursor antibody for bevacizumab (A4.6.1) enhances tumor uptake of irinotecan.[44]
Clinical Implications of Angiogenic Blockade
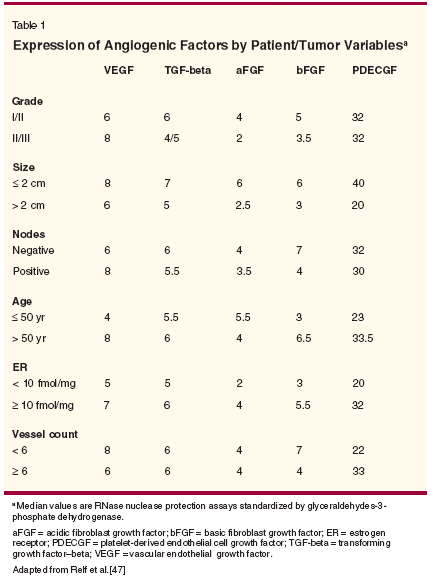
Since angiogenesis in adults is relatively quiescent, antiangiogenic therapy should carry relatively few toxicities, which one would expect to be confined to interference with wound healing and female reproduction and not to overlap with classic nonspecific adverse effects of con ventional agents. Bevacizumab has shown good tolerability in clinical trials, with no discernible adverse effects on wound healing or bleeding, minimal effects on ovulation, and manageable hypertension. Dosing and scheduling remain to be optimized, but as Olszewski and colleagues point out, this is not a straightforward issue of attaining a maximum tolerated dose. The latter concept does not apply to VEGF inhibitors like bevacizumab because dose-limiting toxicity may not be reached, and long-term continuous administration may be required. Therefore, it is still uncertain what constitutes an optimal biologic dose with maximal antiangiogenic efficacy. This issue is further complicated as a reliable biomarker of activity has yet to be determined. There may be different and/or multiple markers for different tumors, disease stages, and even for different patients. Imaging to determine tumor microvascular density and plasma VEGF levels are both under consideration, but neither has so far proved to be a universally accurate indicator. Assessment of efficacy and choice of associated end points may also need to be reconsidered. Rather than using similar measures to those conventionally applied to classic chemotherapeutic regimens, parameters such as time to progression, and disease stabilization may be more appropriate when evaluating antiangiogenic agents, which de facto block further neovascularization and, therefore, tumor growth, but cause little if any tumor regression in established neoplasms, especially in a relatively short time frame such as currently typifies most clinical trials in patients with solid tumors. Scheduling and timing of bevacizumab administration are critical considerations in order to optimize both its antivascular effects and drug delivery to tumor tissue of coadministered chemotherapy. In more advanced cancer, there is undoubtedly a "therapeutic window" between optimal devascularization and normalization by antiangiogenic blockade, and retention of sufficient patent vascular supply to allow adequate tumor saturation with cytotoxic agents. The fact that normalization of tumor vasculature also improves delivery of vital nutrients and oxygen to the tumor cannot be overlooked, and cytotoxic agents need to be administered and timed accordingly.[45] However, given that adequate tumor oxygenation is a prerequisite for effective radiotherapy, the equation is clearly a complex one. Early administration of agents such as bevacizumab may circumvent some of these challenges, both by arresting tumor vascularization (and thus progression) in the dormant or immediately post- dormant state[46] and therefore also by precluding the necessity for more aggressive conventional treatment, at least initially. Evidence suggests that in some early-stage tumors, VEGF may be the only proangiogenic factor being secreted, and thus there is less likelihood of any antiangiogenic agent being "overwhelmed" by a complex cocktail of tumor-promoting growth factors such as arises in late-stage disease (see Table 1).[47] A similar principle applies to adjuvant use of antiangiogenic blockade, or perhaps even its use in a neoadjuvant setting to reduce tumor vascular burden prior to surgery (assuming that operable tumors are synonymous with relatively early-stage disease), and there is a growing body of clinical data to support this. Finally, there is increasing evidence from animal studies suggesting that metronomic administration of low-dose chemotherapy combined with antiangiogenic inhibition has enhanced efficacy, even against late-stage tumors, and that this rationale merits exploration in cancer patients.[48,49] Both chemotherapy and radiotherapy are known to induce VEGF expression, and this may contribute to resistance to conventional treatments, as well as to other biologic interventions.[ 50] Inhibition of VEGF potentiates radiation-mediated killing of tumor cells,[34] and indicators of synergistic efficacy between bevacizumab, conventional chemotherapy, and other targeted agents, was apparent in preclinical studies. Therefore, combining various treatment modalities to target multiple pathways responsible for cancer development and progression may be the way forward for maximal clinical benefit, and such studies are currently ongoing for bevacizumab.
Disclosures:
Dr. Marshall is a speaker and consultant for and receives research support from Roche, Sanofi, Pfizer, Genentech, and Boehringer Ingelheim.
References:
1. Folkman J: Tumor angiogenesis: Therapeutic implications. N Engl J Med 285:1182- 1186, 1971.
2. Rosen LS: Clinical experience with angiogenesi signaling inhibitors: Focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control 9(suppl):36-44, 2002.
3. Ferrara N: Vascular endothelial growth factor as a target for anticancer therapy. The Oncologist 9(suppl 1):2-10, 2004.
4. Black WC, Welch HG: Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med 328:1237-1243, 1993.
5. Kerbel RS: Tumor angiogenesis: Past, present and the near future. Carcinogenesis 21:505-515, 2000.
6. Holash J, Maisonpierre PC, Compton D, et al: Vessel co-option, regression and growth in tumors mediated by angiopoietins and VEGF. Science 284:1994-1998, 1999.
7. Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 9:669-676, 2003.
8. Park JE, Chen HH, Winer J, et al: Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269:25646- 25654, 1994.
9. Alon T, Hemo I, Itin A, et al: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024-1028, 1995.
10. Gerber HP, McMurtrey A, Kowalski J, et al: Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/ KDR activation. J Biol Chem 273:30336- 30343,1998.
11. Pepper MS, Ferrara N, Orci L, et al: Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun 181:902- 906, 1991.
12. Unemori EN, Ferrara N, Bauer EA, et al: Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol 153:557-562, 1992.
13. Lamoreaux WJ, Fitzgerald ME, Reiner A, et al: Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res 55:29-42, 1998.
14. Rousseau S, Houle F, Huot J: Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc Med 10:321-327, 2000.
15. Asahara T, Takahashi T, Masuda H, et al: VEGF contributes to postnatal neovascularization by mobilizing bone marrowderived endothelial progenitor cells. EMBO J 18:3964-3972, 1999.
16. Shen BQ, Lee DY, Gerber HP, et al: Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J Biol Chem 273:29979-29985, 1998.
17. Masood R, Cai J, Zheng T, et al: Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood 98:1904-1913, 2001.
18. Harmey JH, Bouchier-Hayes D: Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: Implications for antiangiogenic therapy. Bioessays 24:280-283, 2002.
19. Tran J, Master Z, Yu JL, et al: A role for surviving chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA 99:4349-4354, 2002.
20. Jain RK: Tumor angiogenesis and accessibility: Role of vascular endothelial growth factor. Semin Oncol 29(suppl 16):3-9, 2002.
21. Carmeliet P: Angiogenesis in health and disease. Nat Med 9:653-660, 2003.
22. Kerbel RS: Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays 13:31-36, 1991.
23. Paley PJ, Staskus KA, Gebhard K, et al: Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer 80:98-106, 1997.
24. Kraft A, Weindel K, Ochs A, et al: Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 85:178-187, 1999.
25. Fujisaki K, Mitsuyama K, Toyonaga A, et al: Circulating vascular endothelial growth factor in patients with colorectal cancer. Am J Gastroenterol 93:249-252, 1998.
26. Saito H, Tsujitani S, Ikeguchi M, et al: Relationship between the expression of vascular endothelial growth factor and the density of dendritic cells in gastric adenocarcinoma tissue. Br J Cancer 78:1573-1577, 1998.
27. Hlatky L, Hahnfeldt P, Folkman J: Clinical application of antiangiogenic therapy: Microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 94:883-893, 2002.
28. Rak J, Yu JL, Klement G, et al: Oncogenes and angiogenesis: Signaling threedimensional tumor growth. J Investig Dermatol Symp Proc 5:24-33, 2000.
29. Shweiki D, Itin A, Soffer D, et al: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843-845, 1992.
30. Warren RS, Yuan H, Matli MR, et al: Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 95:1789-1797, 1995.
31. Yang JC, Haworth L, Sherry RM, et al: A randomized trial of bevacizumab, an antivascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427- 434, 2003.
32. Willett CG, Boucher Y, di Tomaso E, et al: Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145-147, 2004.
33. Yuan F, Chen Y, Dellian M, et al: Timedependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 93:14765- 14770, 1996.
34. Lee CG, Heijn M, di Tomaso E, et al: Anti-vascular endothelial growth factor treat- ment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 60:5565-5570, 2000.
35. Sweeney CJ, Miller KD, Sissons SE, et al: The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res 61:3369-3372, 2001.
36. Hu L, Hofmann J, Zaloudek C, et al: Vascular endothelial growth factor immunoneutralization plus paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am J Pathol 161:1917-1924, 2002.
37. Rini BI, Halabi S, Taylor J, et al for the Cancer and Leukemia Group B. Cancer and Leukemia Group B 90206: A randomised phase III trial of interferon-a or interferon-a plus antivascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma. Clin Cancer Res 10:2584-2586, 2004.
38. Sandler A, Blumenschein GR, Henderson T, et al: Phase I/II trial evaluating the anti-VEGF MAb bevacizumab in combination with erlotinib, a HER1/EGFR-TK inhibitor, for patients with recurrent non-small cell lung cancer (abstract). Proc Am Soc Clin Oncol 23:127, 2004.
39. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for the treatment of metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
40. Jain RK: Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med 7:987-989, 2001.
41. Netti PA, Roberge S, Boucher Y, et al: Effect of transvascular fluid exchange on pressure- flow relationship in tumors: A proposed mechanism for tumor blood flow heterogeneity. Microvasc Res 52:27-46, 1996.
42. Padera TP, Kadambi A, di Tomaso E, et al: Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296:1883-1886, 2002.
43. Stohrer M, Boucher Y, Stangassinger M, et al: Oncotic pressure in solid tumors is elevated. Cancer Res 60:4251-4255, 2000.
44. Wildiers H, Guetens G, De Boeck G, et al: Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 88:1979-1986, 2003.
45. Jain RK: Delivery of novel therapeutic agents in tumors: Physiological barriers and strategies. J Natl Cancer Inst 81:570-576, 1989.
46. Ramanujan S, Koenig GC, Padera TP, et al: Local imbalance of proangiogenic and antiangiogenic factors: A potential mechanism of focal necrosis and dormancy in tumors. Cancer Res 60:1442-1448, 2000.
47. Relf M, LeJeune S, Scott PA, et al: Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 57:963-969, 1997.
48. Bergers G, Hanahan D: Combining antiangiogenic agents with metronomic chemotherapy enhances efficacy against late-stage pancreatic islet carcinomas in mice. Cold Spring Harb Symp Quant Biol 67:293-300, 2002.
49. Klement G, Baruchel S, Rak J, et al: Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 105:R15-24, 2000.
50. Castilla MA, Caramelo C, Gazapo RM, et al: Role of vascular endothelial growth factor (VEGF) in endothelial cell protection against cytotoxic agents. Life Sci 67:1003- 1013, 2000.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.