Combined-Modality Treatment for Operable Pancreatic Adenocarcinoma
Although in centers where pancreatectomy is performed frequently,associated morbidity and mortality rates have improved, long-term outcomesin pancreatic adenocarcinoma patients remain poor when surgeryis the sole therapeutic modality. The impact of adjuvant chemotherapyon survival in patients with localized pancreatic cancer remainsincompletely defined. The European Study Group for Pancreatic Cancer(ESPAC)-1 trial has suggested that overall survival rates are superiorwhen chemotherapy is added to surgery, even when regimens believedto be relatively ineffective in the treatment of advanced diseaseare used. The role of radiotherapy given with chemotherapy is alsounresolved, but chemoradiation continues to receive consideration inthe multimodality approach to localized pancreatic cancer. Enhancedcollaboration and increased involvement by pancreatic surgeons havehelped in the recruitment of pancreatic cancer patients for large-scalerandomized clinical trials in Europe and the United States. Many newerchemotherapeutic agents with efficacy in gastrointestinal cancers haveyet to be investigated in the adjuvant and neoadjuvant settings.
Although in centers where pancreatectomy is performed frequently, associated morbidity and mortality rates have improved, long-term outcomes in pancreatic adenocarcinoma patients remain poor when surgery is the sole therapeutic modality. The impact of adjuvant chemotherapy on survival in patients with localized pancreatic cancer remains incompletely defined. The European Study Group for Pancreatic Cancer (ESPAC)-1 trial has suggested that overall survival rates are superior when chemotherapy is added to surgery, even when regimens believed to be relatively ineffective in the treatment of advanced disease are used. The role of radiotherapy given with chemotherapy is also unresolved, but chemoradiation continues to receive consideration in the multimodality approach to localized pancreatic cancer. Enhanced collaboration and increased involvement by pancreatic surgeons have helped in the recruitment of pancreatic cancer patients for large-scale randomized clinical trials in Europe and the United States. Many newer chemotherapeutic agents with efficacy in gastrointestinal cancers have yet to be investigated in the adjuvant and neoadjuvant settings.
Pancreatic adenocarcinoma acccounts for approximately 32,000 deaths per year in the United States and more than 210,000 deaths per year worldwide.[1,2] The disease is characterized by early spread to the regional lymph nodes and the liver. The majority of patients who appear to have localized, operable pancreatic cancer have subclinical liver metastases at the time of diagnosis, even when modern staging studies reveal no evidence of extrapancreatic disease. Outcome is dictated primarily by the extent of disease and performance status at presentation. Pretreatment classification of tumors as localized, locally advanced, or metastatic is essential for treatment planning. The median survival durations for these clinical stages of pancreatic cancer are 11 to 18 months, 10 to 12 months, and 5 to 7 months, respectively.[3] This review examines the oncologic issues related to pretreatment staging, surgical management, and pre- and postoperative adjuvant therapy, with specific emphasis on the interpretation of recent clinical trials. Pretreatment StagingImaging Studies
- Computed Tomography-Multidetector, multiphase helical computed tomography (CT) is the foundation for clinical staging of pancreatic cancer. Although helical CT is widely available, accurate interpretation and reporting of the tumor-related find- ings remain inconsistent. For optimal pretreatment staging and assessment of operability, a CT report for a patient with a suspected pancreatic or periampullary malignancy should include the following information: (1) Commentary on the presence or absence of a primary tumor in the pancreas or periampullary region;
(2) Commentary on the presence or absence of peritoneal and hepatic metastases;
(3) Description of the patency of the superior mesenteric vein (SMV) and portal vein (PV) and the relation- ship of these veins and their venous tributaries to the tumor
(4) Description of the relationship of the tumor to the superior mesenteric artery (SMA), celiac axis, hepatic artery, and gastroduodenal artery
(5) Commentary on the presence or absence of aberrant vascular anatomy If a CT study performed early in the diagnostic evaluation is of insufficient quality to assess these issues, a new CT study using a pancreatic tumor scanning protocol[4] should be performed. Specific, objective radiographic criteria should be used to assess operability. These objective criteria include (1) no extrapancreatic disease, (2) a patent SMV and PV, and (3) a definable tissue plane between the tumor and regional arterial structures including the celiac axis and SMA.[5] Using helical CT staging and objective criteria for assessment of resectability, many centers have reported resectability rates of 75% to 80% at surgery.[6,7] Thus, a single imaging study with accurate interpretation and use of objective criteria can facilitate preoperative assessment of resectability in most patients. - Laparoscopy-The role of laparoscopy in staging remains undefined. Direct inspection of the peritoneal surface and liver with laparoscopy allows for the identification of metastatic disease, the volume of which is below the threshold for detection by helical CT. Laparoscopy detects helical-CT-occult disease in 4% to 15% of patients.[8] Given the relatively low incidence of positive findings at laparoscopy, most centers utilize this technique selectively in patients who have CT and laboratory findings suggestive but not diagnostic of more advanced disease. Such findings include small volume, low-density liver lesions, low-volume ascites, marked elevation in CA 19-9, or a relatively poor performance status suggestive of more advanced disease.
- Positron-Emission Tomography-The role of positron-emission tomography (PET) in pretreatment staging also remains undefined. Following two reports suggesting that results of 18-fluorodeoxyglucose PET altered clinical management in as many as 43% of patients with pancreatic cancer,[9,10] other reports have failed to confirm this degree of clinical benefit, suggesting instead that PET does not significantly change the clinical approach in most patients staged by helical CT.[11,12] As a consequence, the utility of PET in staging patients with no extrapancreatic disease on helical CT and patients with suspicious but nondiagnostic extrapancreatic CT findings remains unclear. The routine use of PET is also limited by its relatively high cost (relative to other diagnostic tests) and its low specificity for distinguishing between neoplastic and inflammatory causes of distal bile duct obstruction.
CA 19-9
CA 19-9 is a tumor-associated antigen that is frequently elevated in the serum of patients with pancreatic adenocarcinoma.[ 13,14] CA 19-9 may also be mildly elevated in certain benign conditions such as pancreatitis, and thus modest serum CA 19-9 elevations cannot be used for the diagnosis of or screening for pancreatic malignancy. In addition, patients lacking the Lewis antigen glycosyltransferase are unable to produce CA 19-9; this deficiency is present in 7% to 10% of the general population.[13,15] Furthermore, the production of CA 19-9 by normal biliary ductal cells is increased in the settings of biliary obstruction and inflammation; serum CA 19-9 levels are influenced by the presence of jaundice. Since the initial serum CA 19-9 level is obtained soon after presentation with clinical jaundice in many patients, CA 19-9 measurements should be repeated after the biliary obstruction and jaundice have been relieved. There is no consensus on what CA 19-9 level is sufficiently reliable for the diagnosis of cancer in patients in whom there is a clinical suspicion of pancreatic adenocarcinoma. In an analysis by Forsmark and colleagues, serum CA 19-9 levels above 90 U/mL and above 200 U/mL had estimated accuracy rates of 85% and 95%, respectively, in the diagnosis of malignancy in the clinical setting of a suspected pancreatic neoplasm.[16] In one study, the combination of CT and CA 19-9 measurement in nonicteric patients yielded a positive predictive value of 99% to 100% in the diagnosis of pancreatic cancer when a CA 19-9 reference value of 100 to 120 U/mL was used,[17] and a report by Tian et al. suggested that an elevation in CA 19-9 above 750 U/mL was associated with a high probability of locally advanced or metastatic disease.[18] As with most tumor markers, CA 19-9 may be optimally used as supporting clinical evidence in the diagnosis and staging of pancreatic cancer. Pretreatment Biopsy The role of pretreatment biopsy in patients who present with localized disease and in whom surgery is anticipated depends on specific patient and physician factors. One school of thought maintains that for patients presenting with a clinical and radiographic picture consistent with a resectable pancreatic neoplasm, the surgical resection should be both diagnostic and therapeutic.[19] This approach assumes that positive intraoperative biopsies are not required before proceeding with pancreatic resection and that diagnostic pancreatectomy can be performed with low morbidity and mortality rates. A second school of thought espouses reasonable efforts to achieve a pretreatment tissue diagnosis. This approach seeks to separate the diagnostic and therapeutic phases of pancreatic cancer management because doing so may have specific advantages for patients and physicians.[20] A pretreatment biopsy diagnosis allows the patient to consider referral to a regional center specializing in pancreatic cancer treatment. In contrast, when surgery for both diagnosis and treatment is offered, the patient is usu- ally not able to investigate options for further staging or for referral until after surgical staging with or without tumor resection. At this point, an important therapeutic bridge has been crossed.
As outlined below, consideration of referral to a regional center is well founded given the significant differences in operative mortality and longterm survival observed between higher volume regional centers and smaller hospitals. Fine-needle aspiration guided by CT or endoscopic ultrasonography is a relatively safe and accurate means of pretreatment tissue diagnosis.[21] PancreatectomyTechnique
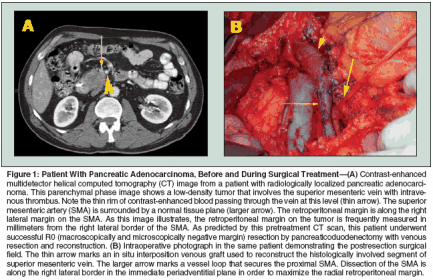
Subtotal pancreatic resection can consist of a left (distal) pancreatectomy or right pancreatectomy, which is usually anatomically described as a pancreaticoduodenectomy or Whipple procedure. For most malignant tumors arising in the pancreatic head and uncinate process, pancreaticoduodenectomy is required to achieve microscopically negative pancreatic parenchymal and retroperitoneal surgical margins. Standard pancreaticoduodenectomy involves resection of the distal stomach, distal bile duct (with cholecystectomy), duodenum, pancreatic head and uncinate process, proximal jejunum, and regional lymph nodes.[22] Gastrointestinal reconstruction following pancreaticoduodenectomy requires enteric, biliary, and pancreatic anastomoses, usually by gastrojejunostomy (or duodenojejunostomy in the case of pylorus-preserving pancreaticoduodenectomy), choledochojejunostomy, and pancreaticojejunostomy (or pancreaticogastrostomy). Technical modifications that have no clearly demonstrated advantage or adverse oncologic impact include pylorus preservation[23] and extended lymphadenectomy.[24,25] Randomized trials of pancreaticoduodenectomy with extended vs standard lymphadenectomy have demonstrated increased morbidity with the extended dissection but no improvement in survival rates.[24,25] As a consequence, most Western surgeons perform either a standard or pyloruspreserving pancreaticoduodenectomy with a standard lymphadenectomy (including peripancreatic lymph nodes) but not extended regional lymphadenectomy. The most oncologically significant and technically demanding step in pancreaticoduodenectomy is the retroperitoneal vascular dissection along the proximal SMA. This step occurs during the final phase of the tumor resection, after division of the stomach, jejunum, bile duct, and pancreas. This step can be technically difficult and associated with a risk of hemorrhage from the pancreaticoduodenal arteries arising from the right posterolateral side of the SMA. In an effort to minimize this risk and save time, many surgeons do not fully mobilize the SMV to adequately expose the SMA and instead choose an easier dissection plane several millimeters to the right of the SMA. Unfortunately, this compromises the radial retroperitoneal margin, which is of critical oncologic significance. Pancreaticoduodenectomy with segmental resection of the SMV or PV is necessary when the tumor is inseparable from the lateral wall of the SMV or PV.[26] When intraoperative findings suggest venous involvement, approximately 80% of patients undergoing pancreatectomy with venous resection have histologic evidence of tumor extension into the vein wall.[26] However, venous resection should be performed only in carefully selected patients who have tumor adherence to the SMV or PV but have no evidence of tumor extension to the SMA or celiac axis (Figure 1). Following pancreaticoduodenectomy with venous resection, patients with pathologic invasion of the SMV or PV do not have an increased frequency of surgical margin or lymph node positivity compared to patients without involvement of the SMV or PV, and patient survival after pancreaticoduodenectomy with venous resection is comparable to that observed in patients with similar tumors that do not require venous resection.[27] Hospital Pancreatectomy Volume and Mortality Rates
Several reports from Europe[28,29] and North America[30-32] have established a clear relationship between institutional pancreatectomy volume and perioperative mortality rates. For North American patients, the recent analyses of national databases by Birkmeyer et al[30] and Kotwall et al[31] of 10,507 and 25,000 patients who underwent pancreatectomy are among the most compelling of these reports. Birkmeyer and colleagues demonstrated that patients undergoing pancreatectomy at institutions with very low (< 1 pancreatectomy per year), low (1 or 2 per year), or moderate (3-5 per year) volumes had mortality rates of 16.2%, 14.4%, and 10.9%, respectively. In comparison, patients undergoing pancreatectomy at higher-volume (> 16 per year) institutions had a mortality rate of only 3.9%.[30] Kotwall et al reported similar findings in an analysis of surgical volume and postoperative mortality rates at hospitals categorized as rural, urban nonteaching, and urban teaching. In that report, the overall national operative mortality rate for pancreaticoduodenectomy was 14%, and there was a clear relationship between perioperative and postoperative mortality rates and hospital surgical volume and category.[31] Data from these two reports demonstrate that the majority of patients undergoing pancreatic resection in the United States have surgery in hospitals where the pancreatectomy volume is low or moderate and the mortality rate is in excess of 10%. Higher-volume hospitals also have improved long-term cancer-related outcomes. Birkmeyer et al performed a retrospective cohort study of all 7,229 patients over age 65 years who underwent pancreaticoduodenectomy in the United States between 1992 and 1995.[33] The 3-year overall survival rates were higher at high-volume centers (37%) than at moderate- (29%), low- (26%), and very lowvolume hospitals (25%, P < .001). After exclusion of perioperative deaths and adjustment for case mix, patients who underwent surgery at high-volume centers remained less likely to experience late deaths than did patients at very low-volume hospitals (adjusted hazard ratio [HR] = 0.69; 95% confidence interval [CI] = 0.62- 0.76). The reasons for this apparent improvement in long-term outcome are not evaluable from this type of analysis of administrative databases but are presumed to be related to improved staging and possibly increased use of adjuvant treatments in these patients. The data establishing a definite relationship between institutional volume and short- and long-term survival rates suggest that a public health policy promoting regionalization of care for patients with operable pancreatic cancer would immediately improve survival rates.[34,35] Indeed, the survival advantages associated with regionalization of care may approach or exceed those available with current adjuvant treatments.[35] Complications and Postpancreatectomy Recovery
Although mortality rates for pancreaticoduodenectomy have declined to less than 3% in high-volume centers,[36-38] the morbidity of pancreatectomy remains high, with pancreatic leak and infection among the most common surgical complications.[ 39] Postpancreatectomy recovery can be prolonged owing to the complex interplay between comorbid factors and surgical morbidity. Unfortunately, prolonged recovery following pancreatectomy may delay and in some cases prevent planned adjuvant treatment. Reports from major referral centers for pancreatic surgery demonstrate that approximately 25% of postpancreatectomy patients do not recover satisfactorily (ie, achieve a good enough performance status) to consider postoperative chemoradiation.[ 40,41] However, this figure should be considered a minimum estimate of the impact of delayed postoperative recovery, as the majority of patients in North America undergo pancreatectomy at low- to intermediate- volume institutions, where morbidity (as inferred from mortality data) and mortality rates are considerably higher than at regional centers.[30,31] Many medical and radiation oncologists have little familiarity with normal postpancreatectomy recovery and the specific intermediate-term complications that can affect a patient's performance status at the time of assessment for adjuvant treatment. For patients under age 70 with minimal comorbidity, at least 6 to 8 weeks is generally required for recovery to a performance and nutritional status that is satisfactory for consideration of postoperative treatment. Clinically significant issues that are frequently present in variable degrees of severity at the time of consultation for adjuvant treatment include low-grade delayed gastric emptying, as manifested by occasional nausea or vomiting, and pancreatic exocrine insufficiency, manifested by frequent bowel movements with bulky or greasy stools and difficulty maintaining or gaining weight. A trial of metoclopramide is often considered for patients with clinical evidence of delayed gastric emptying. In addition, pancreatic enzyme supplements should be used in almost all patients. These enzyme supplements should be titrated to reduce bowel movement frequency to one or two per day. H2-receptor blockers or proton pump inhibitors should also be used in all patients to minimize the risk for marginal ulceration at the gastrojejunal anastomosis, prevent treatment- related gastritis if radiotherapy is planned, and facilitate the action of pancreatic exocrine supplements, which require an alkaline environment for activation. Classification and Oncologic Significance of Surgical Margins
For tumors arising in the pancreatic head and uncinate process, the retroperitoneal margin is defined anatomically as the margin between the medial edge of the tumor and the right lateral border of the SMA (Figure 1). During pancreaticoduodenectomy, dissection must occur in the immediate periadventitial plane of the SMA to maximize the radial retroperitoneal margin. As a consequence, the SMA dissection is the most oncologically significant step in pancreaticoduodenectomy. Difficulties in maximizing and pathologically evaluating the retroperitoneal margin include the technical challenge of safe dissection along the proximal SMA and the fact that identification and orientation of the specimen for accurate margin assessment is usually possible only at the time of surgery by combined efforts of the surgeon and surgical pathologist. This margin should be inked in the surgical suite, and the pathology report should describe the distance in millimeters between the tumor and the inked retroperitoneal margin; retrospective evaluation of this margin is not possible. Details of the procedure for margin assessment are outlined in the sixth edition of the AJCC Cancer Staging Manual.[42] Pancreatectomy margins (especially the retroperitoneal margin) should be classified by the surgeon after integration of the operative findings and the microscopic surgical margin findings in the final pathology report. All pancreatic resections should be class- ified according to residual disease status (termed "R" factor): R0, no gross or microscopic residual disease; R1, microscopic residual disease (microscopically positive surgical margins with no gross residual disease); and R2, grossly evident residual disease. The pathologist cannot usually differentiate an R1 (microscopically positive) from an R2 (grossly positive) retroperitoneal margin in the absence of information regarding the retroperitoneal dissection, which should be included in the operative note. The R designation should appear in the final pathology report and should be consistent with the dictated operative note. For example, if the surgeon states that gross tumor was encountered when completing the retroperitoneal dissection, a positive histologic margin should result in the R2 designation in the final pathology report. In the absence of this information being included in the operative report, the proper R designation cannot be determined. The difficulty in differentiating R1 from R2 resections has significant implications for clinical trials examining the potential survival advantage of adjuvant or neoadjuvant therapies.
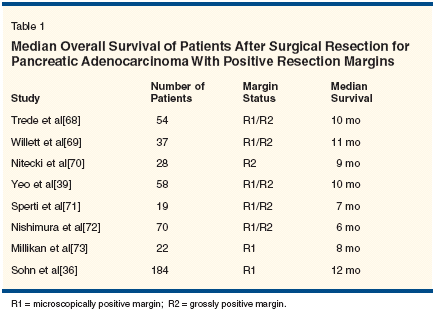
The limited data available suggest that the radial retroperitoneal margin is positive in 15% to 25% of patients after pancreaticoduodenectomy (single- institution data).[43] The clinical significance of a positive margin is indicated in Table 1. Surgical resection with a grossly positive surgical margin is associated with a median survival of 6 to 12 months. This median survival in patients with ostensibly localized pancreatic cancer is comparable to that observed in patients with locally advanced pancreatic cancer treated nonsurgically.[ 44,45] Thus, a positive-margin (R2) pancreatic resection represents a suboptimal therapeutic result. Because of the lack of accurate historical recording of R status, the impact of an R1 resection-especially in the era of contemporary adjuvant therapy-is unknown. Oncologic Outcome After Pancreatectomy Alone
Unfortunately, despite improvements in the short-term outcomes of patients undergoing pancreatic resection for pancreatic cancer, there has been no significant improvement in oncologic outcome over the past decade when pancreatectomy is used as the sole therapeutic modality. Many surgical series have relatively short median follow-ups and/or do not report sufficient information on the use of nonsurgical treatment to draw firm, accurate conclusions regarding outcome in patients treated by pancreatectomy alone. However, retrospective reports of surgical outcome stratified by additional treatments and results from the pancreatectomy-alone arm of prospective randomized trials of pancreatectomy alone vs pancreatectomy plus postoperative chemoradiation suggest that the median survival following pancreatectomy alone (without chemoradiation) for localized pancreatic adenocarcinoma ranges from 11 to 17 months (Table 2).[46-49]
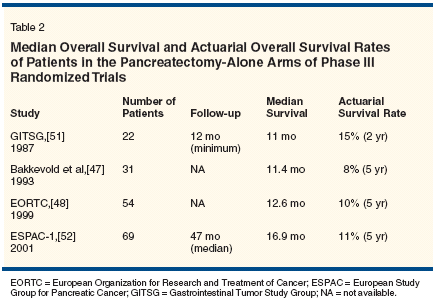
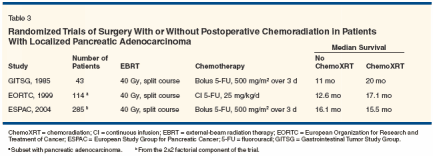
That said, the use of contemporary pretreatment imaging and improved patient selection may increase the median survival a few months over these historical estimates. For purposes of treatment comparisons and protocol planning, median survival durations of 12 to 18 months and 5-year actuarial overall survival rates of 10% to 15% with pancreatectomy alone appear to be accurate estimates. On this basis, it is clear that for most patients, pancreatectomy is necessary but not sufficient for cure of pancreatic cancer.Adjuvant Chemotherapy Pancreatic cancer metastasizes early during its natural course, and despite surgical intervention with curative intent, most patients will ultimately die with disseminated dis- ease. The majority of patients who present with potentially resectable tumors must therefore harbor occult metastatic cancer. Over the past 2 decades, studies of adjuvant chemotherapy for cancers of the rectum, colon, and, most recently, stomach have proven that systemic therapy directed at microscopic metastases enhances the chances of disease-free and longterm survival for patients at high risk of relapse after surgery. While chemotherapy has quite modest benefits in the treatment of advanced pancreatic cancer (which is more drug-resistant than other gastrointestinal tumors), such treatment continues to be incorporated into trials of adjuvant and neoadjuvant therapy for resected pancreatic cancer. Until recently, only limited information about the role of chemotherapy for operable pancreatic cancer was available, and most adjuvant-therapy trials focused on the role of combinedmodality therapy for resected pancreatic cancer. However, systemic chemotherapy has been studied as an adjunct to surgery in these patients. In a small randomized trial conducted by Bakkevold et al, 61 curatively resected patients with either pancreatic cancer (n = 47) or ampullary cancer (n = 14) were randomly assigned to observation alone or fluorouracil (5-FU), doxorubicin, and mitomycin (Mutamycin).[47] The median survival was longer in the group of patients receiving chemotherapy (23 vs 11 months for the observation-only group; P = .02), but no long-term survival advantage was observed. In a larger Japanese trial that enrolled patients with a variety of hepatobiliary tract tumors including pancreatic cancer, patients were randomized after curative resection to a regimen of 5-FU and mitomycin or to observation.[50] The 5-year overall survival was 18% for patients undergoing surgery alone but 11% for patients undergoing surgery and postoperative chemotherapy. The trend in most adjuvant-therapy trials under way now is to move beyond 5-FU alone (particularly bolus 5-FU) and to investigate the use of gemcitabine or prolonged infusions of 5-FU combined with other drugs. Since gemcitabine has shown superiority to 5-FU against advanced disease, current randomized trials are testing it in the adjuvant-therapy setting. In Europe, where a role for radiotherapy in operable pancreatic cancer is considered a less compelling question than comparisons of different adjuvant chemotherapy regimens (see discussion of the European Study Group for Pancreatic Cancer [ESPAC]-1 trial below), gemcitabine will be compared to bolus 5-FU and leucovorin. ESPAC-3 is a multicenter European trial that plans to enroll more than 900 patients. Patients who recover adequately from surgery will be randomized to receive gemcitabine (1,000 mg/m2 over 30 minutes, weekly for 3 weeks, every 28 days) for 6 months or bolus 5-FU and leucovorin for 6 months. In North America, where radiation is still considered to be an important component of adjuvant therapy, the role of gemcitabine is also being investigated. In July 1998, the Radiation Therapy Oncology Group (RTOG) activated the first American phase III cooperative group study of postoperative adjuvant therapy for resected pancreatic adenocarcinoma (RTOG 9704) since the Gastrointestinal Tumor Study Group (GITSG) trial. Patients were randomly assigned to receive weekly gemcitabine or infu- sional 5-FU, given before and after 5-FU-based chemoradiation. The study aims to determine any significant differences between gemcitabine and infusional 5-FU given as systemic therapy for microscopic metastatic disease. The trial has reached its accrual goals, and preliminary survival results are anticipated in 2005. Adjuvant Chemoradiation Treatment Chemoradiation is used to reduce the probability of local tumor recurrence in patients who undergo potentially curative resection of pancreatic cancer. Based on limited data,[1-5] postoperative adjuvant chemoradiation is frequently used in the United States. This practice is in part based on the favorable results of a single randomized trial of postoperative adjuvant chemoradiation vs surgical resection alone, conducted by the GITSG.[46] In this study, 43 patients who had successfully recovered from pancreaticoduodenectomy were entered in the study over 8 years. In the adjuvant chemoradiation arm, radiotherapy was delivered to the primary disease site and regional lymphatics in a split course of 40 Gy over 6 weeks with concurrent 5-FU (500 mg/m2/d by intravenous bolus on days 1 to 3 of each 2-week radiotherapy course). Weekly maintenance 5-FU was then given for 2 years or until disease recurrence. The results in the chemoradiation arm were significantly superior to the results in the observation arm (43% vs 18% at 2 years and 14% vs 5% at 5 years, P < .05).[46] An additional 30 patients were subsequently entered in the trial with a duplication of the results.[51]
- EORTC Trial-In contrast are findings reported by the European Organization for Research and Treatment of Cancer (EORTC).[48] Between 1987 and 1995, 218 patients who had undergone pancreaticoduodenectomy for adenocarcinoma of the pancreas or periampullary region were randomized to receive either chemoradiation (40 Gy in a split course along with 25 mg/kg/d of 5-FU given as a continuous infusion during radiotherapy) or no further treatment. The median overall survival duration was 24.5 months for the group who received adjuvant chemoradiation and 19 months for the group who received surgery alone (P = .2). As to patients with pancreatic cancer, the median overall survival duration was 17.1 months for those who received adjuvant therapy and 12.6 months for those who received surgery alone (P = .099). Twenty percent of 104 evaluable patients assigned to receive chemoradiation did not receive the intended therapy because of patient refusal, medical comorbidities, or rapid tumor progression. Thus, the low level of compliance and inadequate statistical power of this trial could explain the lack of a clear benefit. Furthermore, this study, as well as the GITSG study, used a suboptimal chemotherapy dose and schedule and an inadequate radiotherapy dose, equipment (supervoltage), and schedule (split course).
- ESPAC-1 Trial-The ESPAC also evaluated postoperative chemoradiation using a GITSG-like treatment regimen in a multi-institutional, randomized trial.[52] The ESPAC-1 trial was designed to evaluate the independent effects of chemotherapy and chemoradiation. Patients with resected pancreatic adenocarcinoma were randomly assigned to one of four treatment groups in a 2*2 factorial design: (1) observation only, (2) chemoradiation using the GITSG regimen of 40 Gy delivered in a split course plus 5-FU (500 mg/m2/d) given by intravenous bolus on days 1 to 3 of each radiation course, (3) 5-FU (425 mg/m2/d) and leucovorin (20 mg/m2/d) given by intravenous bolus on 5 consecutive days every 28 days for six cycles, and (4) both chemoradiation and chemotherapy administered as described above. From 1994 to 2000, 53 medical centers in 11 countries entered 289 patients in the ESPAC-1 trial. Grade 3 or 4 toxicities were reported in only 29 patients (10%); 2 patients who received chemoradiation died of treatment- related toxicities. The median follow-up among surviving patients was 47 months. The median overall survival durations for patients randomized to observation, chemoradiation, chemotherapy, and chemoradiation plus chemotherapy were 16.9 months, 13.9 months, 21.6 months, and 19.9 months, respectively. The trial did not have the statistical power to compare these median survivals directly. However, the median overall survival among the 145 patients randomized to receive chemoradiation (with or without additional chemotherapy) was 15.9 vs 17.9 months in the 144 patients not assigned to receive chemoradiation (HR for death = 1.28; 95% CI = 0.99-1.66; P = .05). The median overall survival among the 147 patients randomized to receive chemotherapy was 20.1 vs 15.5 months among the 142 patients who received observation or chemoradiation only (HR for death = 0.71; 95% CI = 0.55-0.92; P = .009). However, only 61 (41%) of the 147 patients received all intended chemotherapy, and the survival duration of those who did and did not receive intended therapy was not provided. Despite these limitations, the ESPAC investigators concluded that adjuvant chemotherapy has a significant survival benefit and that adjuvant chemoradiation had an adverse effect on survival.
- Numerous issues have been raised in the interpretation of the ESPAC-1 findings. An obvious benefit of the prospective randomized trial design is the ongoing monitoring of compliance with protocol-assigned treatment. However, the ESPAC investigators were unable to evaluate compliance (and presumably also toxicity) with assigned therapy in 17% and 12% of the patients assigned to receive chemotherapy or chemoradiotherapy, respectively. In the subset of patients for whom treatment compliance was evaluable, only 70% assigned to receive chemoradiation were treated with protocol-prescribed treatment. Compliance with protocol-assigned chemotherapy was also suboptimal; only 61 (50%) of 122 patients assigned to receive chemotherapy for whom treatment details were available received all intended treatment; no treatment-related information was available for 25 additional patients assigned to receive protocol-based chemotherapy. In addition, the survival duration of those who did and did not receive the intended treatment was not provided in the manuscript. If the median survival durations of patients who did and did not receive chemotherapy or chemoradiation are substantially different, concerns regarding the confounding effects of noncompliance would be reduced. Another important issue relates to the implications of the pattern-of-failure data reported. Among the 158 patients who developed disease recurrence, local recurrence (best considered progression of microscopic or macroscopic residual tumor) was a component of failure in 63% of patients. This frequency of local disease progression is considerably higher than the 18% rate of microscopically positive surgical margins reported at protocol entry, suggesting that many patients who were believed to have microscopically negative margins at the time of protocol entry in fact had macroscopically (R2) or microscopically (R1) positive surgical margins. No central quality assurance for surgery, pathology, or pretreatment radiology was incorporated as part of this multicenter trial, and thus it is easy to understand how substantial numbers of patients with incompletely resected disease were inadvertently included in ESPAC-1. As a consequence, the findings of ESPAC-1 may not be readily extrapolated to clinical settings where surgical margin assessment is routinely performed according to the principles outlined by the American Joint Committee on Cancer,[42] the majority (80%) of patients undergo a true R0 resection, and accurate postoperative pretreatment restaging is performed prior to the initiation of adjuvant therapy. The impression that ESPAC-1 included many patients with incompletely resected disease is further supported by comparison of the local recurrence rates reported in ESAPC-1 with those reported in series where R0 and R1 resection rates are accurately reported based on prospective uniform surgical pathology reporting. Indeed, in a consecutive series of 132 patients treated with pancreatectomy and preoperative chemoradiation at our institution,[43] there was a 12% rate of microscopically positive surgical margins-similar to the 18% rate of positive margins reported in ESPAC-1. However, local recurrence rates were 10% in our series vs 63% in ESPAC-1. These pattern-offailure data affect the interpretation of the overall trial findings and clearly demonstrate that quality control for surgery, pathology, and pretreatment radiology is a necessary component of the design of future clinical trials. It is also important to note that the chemoradiation treatment used in ESPAC-1 does not represent state-ofthe- art treatment now or at the time that ESPAC-1 was conceived. The regimen used in ESPAC-1 (40 Gy in two 20-Gy courses with bolus 5-FU) is the same regimen utilized in the 1970s for the first randomized trial of adjuvant chemoradiation performed by the GITSG.[53] Most multidisciplinary groups are now using standard fractionation radiation to a total dose of 45-50.4 Gy with infusional 5-FU (an option available at the time that ESPAC-1 was designed) or, more recently, capecitabine (Xeloda) or gemcitabine. As a result, the ESPAC-1 trial findings are best reviewed as evidence that the chemoradiation regimen tested- intermediate-dose split-course radiation with bolus 5-FU-is not adequate for control of disease in the setting of positive surgical margins. For patients with resected pancreatic cancer and physicians contemplating adjuvant treatment for these patients, it is not clear to what extent the ESPAC findings can be generalized to clinical settings where patients are treated with macroscopically complete tumor resection, accurate pathology staging, and modern chemoradiation consisting of single-course radiation to a dose of 45-50.4 Gy combined with infusional 5-FU, capecitabine, or gemcitabine
- Intergroup Trial-The most commonly used postoperative chemoradiation regimen for pancreatic cancer in the United States is radiotherapy (50.4 Gy in 28 fractions) delivered to the operative bed and regional lymphatics with concurrent 5-FU delivered by protracted intravenous infusion. A field reduction is typically introduced after 45 Gy in 25 fractions, with the tumor bed (including the retroperitoneal margin) being treated with an additional 5.4 Gy in 3 fractions. A multi-institutional assessment of treatment efficacy using modern doses, schedules, equipment, and techniques will be available when the results of the RTOG-led Gastrointestinal Intergroup Protocol 97-04 are reported. In this study, 538 pancreatic cancer patients, after undergoing pancreaticoduodenectomy, were randomized to receive either route gemcitabine dose or protracted intravenous infusion of 5-FU (250 mg/m2/d) before and after concurrent chemoradiation (50.4 Gy). The chemoradiation component of the trial was not a randomized variable, but because locoregional control is one of the end points that will be evaluated, an assessment of the adequacy of the locoregional treatment will be possible. A comparison of the patterns of failure in this study with those in the ESPAC-1 study will be very informative.
Neoadjuvant Chemoradiation There are many practical and theoretical advantages to preoperative adjuvant (ie, neoadjuvant) therapy for localized pancreatic cancer.[54] Foremost among the theoretical advantages is the ability to provide immediate systemic therapy for a disease that is systemic at diagnosis in most patients. A second, more practical advantage is improved patient selection for pancreatic resection-an operation associated with significant patient morbidity even when performed by experienced surgeons. This improved patient selection arises because patients with rapidly progressive systemic disease are identified during the presurgical restaging evaluation performed following neoadjuvant treatment. In prospective studies, 20% to 40% of patients who begin a program of neoadjuvant plus treatment do not undergo planned surgery because of disease progression or development of clinically significant medical comorbidities.[ 43,55] These patients are thus spared the morbidity and risk of perioperative death associated with pancreaticoduodenectomy. However, this practical advantage of neoadjuvant treatment creates challenges in comparing the results obtained with preoperative and postoperative treatment because of the "dropouts" that occur in a program of preoperative treatment. Recent pilot and phase II studies of adjuvant chemoradiation have utilized radiation regimens with shorter courses and higher doses per fraction (termed "rapid fractionation"). These studies demonstrated that a preoperative 30-Gy rapid-fractionation regimen is well tolerated, with a toxicity profile that appears to depend most on the radiation sensitizer used; hospital admission rates were 4%, 11%, and 43% for patients treated with preoperative radiation (30 Gy) and 5-FU,[56] paclitaxel,[57] or gemcitabine,[ 55] respectively. In a retrospective review of the University of Texas M. D. Anderson Cancer Center's experience with neoadjuvant chemotherapy (5-FU or paclitaxel) and radiation (30 or 45- 50.4 Gy) in 132 patients with localized and subsequently resected pancreatic cancer, the median overall survival was 21 months.[43] This compares favorably to the median overall survival of 11 to 13 months reported after pancreatectomy alone, although such comparisons are limited by the selection advantage (bias) associated with preoperative treatment. Retrospective comparisons of patients with localized pancreatic cancer treated with pre- and postoperative chemoradiation have not demonstrated any differences in long-term outcome between these two treatment approaches.[ 40,41] In the absence of randomized clinical trials comparing preoperative chemoradiation and pancreatectomy to pancreatectomy alone, it is unclear whether the apparent differences in median survival duration are related to patient selection, stage migration (arising from improved pretreatment staging in recent years), treatment, or a complex interaction of these and other factors. The results of pilot and phase II trials of preoperative chemoradiation have led to continued interest in neoadjuvant therapy in a limited number of centers. However, while medical and radiation oncologists familiar with preoperative treatment approaches for other solid tumors may see the practical and theoretical advantages of preoperative therapy for localized pancreatic cancer, this strategy is often not well accepted by pancreatic surgeons. Many surgeons are reluctant to submit good-performance-status patients with resectable pancreatic cancer to extended, potentially toxic preoperative treatment programs that could delay surgery. In addition, there are practical challenges associated with preoperative treatment including the requirement for a pretreatment tissue diagnosis and the need for intermediate-term palliation of jaundice using endoscopically placed biliary stents. These stents can occlude, leading to biliary stent-related morbidity in approximately 15% of patients treated with preoperative chemoradiation.[ 58] As a consequence of these practical issues, neoadjuvant chemoradiation remains a conceptually attractive but still investigational treatment for localized pancreatic cancer.
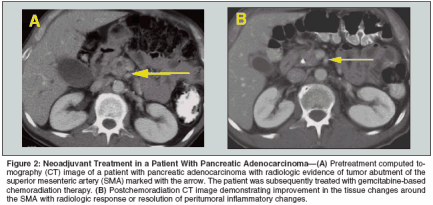
Downstaging Locally Advanced Disease The term "downstaging" is generally used to describe either the reduction of the clinical stage to a lower pathologic stage than expected after neoadjuvant therapy and surgical resection, or the conversion of an unresectable tumor to a resectable one with the use of cytotoxic therapy. In reference to pancreatic cancer, the term downstaging is usually used to describe the latter situation. The interpretation of whether downstaging truly occurs is limited by inconsistent and subjective definitions of resectability and by inadequate preoperative radiologic assessments of resectability. Probably the most variable factor in determining resectability and thus interpreting whether downstaging has occurred is the approach to vascular involvement. Imaging that is not designed to address the issue of vascular involvement may not have adequate resolution for making an assessment. Thus, CT scans of the same patient at different times may result in different interpretations, even without therapy being administered. Although most surgeons agree that tumor encasement of either the celiac artery or the SMA (as seen on CT or MRI) constitutes unresectable disease, opinions vary with regard to more limited arterial involvement. It is probably in the group of patients with limited arterial involvement that, theoretically, active cytotoxic therapy could lead to downstaging. At M. D. Anderson, patients who have locally advanced tumors and have undergone negative-margin resections have typically had very limited arterial abutment (less than one-third of the circumference and less than 1 cm of the length of the artery) and a response to chemoradiation.[59] Such tumors are sometimes referred to as "marginally resectable" or "borderline resectable." In such patients, it is not possible to differentiate actual tumor downstaging from resolution of peritumoral fibrosis and inflammation (Figure 2). Another factor affecting the determination of resectability and the determination of whether downstaging has occurred is the strategy for tumor involvement of the SMV/PV confluence. In the opinion and experience of the authors, tumor extension to a venous structure without occlusion is not an absolute contraindication to resection. Such a vein can be successfully resected and reconstructed at the time of pancreaticoduodenectomy. However, many surgeons would consider this type of tumor extension, seen either intraoperatively or on preoperative imaging, as evidence of un- resectability. Thus, the attribution of increased resectability to chemoradiation in some studies could be simply due to a difference in surgical opinion and practice. In other words, the existence of variable definitions of locally advanced pancreatic cancer gives the impression that clinically meaningful downstaging occurs more commonly than it actually does. Rigidly defined, true downstaging must include an objective definition of resectability as well as reproducible imaging before and after chemoradiation. Although many studies have reported that downstaging in this setting occurred, very few fulfill these criteria. Even with nonrigid criteria, downstaging after 5-FU-based chemoradiation is uncommon. Review of the available literature suggests that a small percentage (8%-16%) of patients with clinically unresectable disease treated with 5-FU-based chemoradiation have eventually undergone negative- margin resection.[60-67] The use of newer radiosensitizers such as paclitaxel and gemcitabine with radiotherapy could result in increased local tumor response and possibly increased resectability rates in patients with locally advanced disease, but this has not been clearly demonstrated. Ideally, all studies using novel chemoradiation regimens should adhere to a strict CTbased definition of locally advanced pancreatic cancer that includes arterial involvement (low-density tumor inseparable from the SMA or celiac axis on contrast-enhanced CT) or occlusion of the SMV/PV confluence when addressing the issue of downstaging. Conclusions Pancreaticoduodenectomy is the primary treatment for patients with localized pancreatic cancer. Using contemporary staging techniques, including helical CT, laparoscopy, and endoscopic ultrasonography, a diagnosis of pancreatic cancer can be established and an R0 resection accomplished in 80% of patients deemed preoperatively to have surgically resectable tumors at high-volume centers. Although there have been improvements in the morbidity and mortality rates for pancreatectomy performed in high-volume centers, long-term oncologic outcomes remain poor when surgery is the sole therapeutic modality. The primary cause of disease progression after pancreaticoduodenectomy is a persistent burden of subclinical disease, both locoregional and systemic. Thus, the current treatment algorithm for localized pancreatic cancer involves surgery as a necessary but insufficient step in most patients. The impact of adjuvant chemotherapy on survival in patients with localized pancreatic cancer remains undefined. There is no consensus on what constitutes "standard" adjuvant chemotherapy. However, the ESPAC-1 trial has suggested that overall survival rates are superior when chemotherapy is added to surgery, even when regimens believed to be relatively ineffective in the treatment of advanced disease are used. Given the systemic nature of pancreatic cancer in most patients, it is ultimately a question of how, rather than if, chemotherapy is best employed. The role of radiotherapy given with chemotherapy, either pre- or postoperatively, is also unresolved. This is especially true at the international level, where significant differences exist in the interpretation of the literature. Given the variable results observed in randomized trials and the challenges in interpreting the ESPAC-1 trial, chemoradiation continues to receive consideration in the multimodality approach to localized pancreatic cancer. Perhaps most important, enhanced collaboration and increased involvement by pancreatic surgeons have helped in the recruitment of pancreatic cancer patients for large-scale randomized clinical trials in Europe and the United States. Many of the newest chemotherapy drugs with efficacy in gastrointestinal cancers (eg, capecitabine, bevacizumab [Avastin], and oxaliplatin [Eloxatin]) have not yet been investigated in the adjuvant and neoadjuvant settings. The possibilities for therapeutic progress against pancreatic cancer have never been greater than they are today.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. International Agency for Research on Cancer (IARC): GLOBOCAN 2002. Available at www-depdb.iarc.fr/globocan/GLOBOframe. htm. Accessed January 24, 2005.
2. Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005.
3. Evans DB, Abbruzzesse JL, Willett CG: Cancer of the pancreas, in Cancer: Principles & Practice of Oncology. New York, Lippincott Williams & Wilkins, 2001.
4. McNulty NJ, Francis IR, Platt JF, et al: Multi-detector row helical CT of the pancreas: Effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology 220:97-102, 2001.
5. Fuhrman GM, Charnsangavej C, Abbruzzese JL, et al: Thin-section contrastenhanced computed tomography accurately predicts the resectability of malignant pancreatic neoplasms. Am J Surg 167:104-111, 1994.
6. Friess H, Kleeff J, Silva JC, et al: The role of diagnostic laparoscopy in pancreatic and periampullary malignancies. J Am Coll Surg 186:675-682, 1998.
7. Barreiro CJ, Lillemoe KD, Koniaris LG, et al: Diagnostic laparoscopy for periampullary and pancreatic cancer: What is the true benefit? J Gastrointest Surg 6:75-81, 2002.
8. Pisters PW, Lee JE, Vauthey JN, et al: Laparoscopy in the staging of pancreatic cancer. Br J Surg 88:325-337, 2001.
9. Rose DM, Delbeke D, Beauchamp RD, et al: 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surg 229:729- 737, 1999.
10. Jadvar H, Fischman AJ: Evaluation of pancreatic carcinoma with FDG PET. Abdom Imaging 26:254-259, 2001.
11. Kasperk RK, Riesener KP, Wilms K, et al: Limited value of positron emission tomography in treatment of pancreatic cancer: Surgeon’s view. World J Surg 25:1134-1139, 2001.
12. Kalady MF, Clary BM, Clark LA, et al: Clinical utility of positron emission tomography in the diagnosis and management of periampullary neoplasms. Ann Surg Oncol 9:799-806, 2002.
13. Ritts RE, Pitt HA: CA 19-9 in pancreatic cancer. Surg Oncol Clin N Am 7:93-101, 1998.
14. Plebani M, Basso D, Panozzo MP, et al: Tumor markers in the diagnosis, monitoring and therapy of pancreatic cancer: State of the art. Int J Biol Markers 10:189-199, 1995.
15. Safi F, Schlosser W, Kolb G, et al: Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. J Gastrointest Surg 1:106-112, 1997.
16. Forsmark CE, Lambiase L, Vogel SB: Diagnosis of pancreatic cancer and prediction of unresectability using the tumor-associated antigen CA19-9. Pancreas 9:731-734, 1994.
17. Ritts RE Jr, Nagorney DM, Jacobsen DJ, et al: Comparison of preoperative serum CA19- 9 levels with results of diagnostic imaging modalities in patients undergoing laparotomy for suspected pancreatic or gallbladder disease. Pancreas 9:707-716, 1994.
18. Tian F, Appert HE, Myles J, et al: Prognostic value of serum CA 19-9 levels in pancreatic adenocarcinoma. Ann Surg 215:350- 355, 1992.
19. Brennan MF: Pancreatic cancer. J Gastroenterol Hepatol 15(suppl):G13-G16, 2000.
20. Evans DB, Wolff RA, Crane CH: Combined modality treatment for pancreatic cancer, in Progress in Oncology. Sadbury, Massachusetts, Jones & Bartlett, 2004.
21. Ihse I, Axelson J, Dawiskiba S, et al: Pancreatic biopsy: Why? When? How? World J Surg 23:896-900, 1999.
22. Evans DB, Pisters PWT, Lee JE: Pancreaticoduodenectomy (Whipple operation) and total pancreatectomy for cancer, in Mastery of Surgery. New York, Lippincott, Williams & Wilkins, 2001.
23. Takao S, Aikou T, Shinchi H, et al: Comparison of relapse and long-term survival between pylorus-preserving and Whipple pancreaticoduodenectomy in periampullary cancer. Am J Surg 176:467-470, 1998.
24. Pedrazzoli S, DiCarlo V, Dionigi R, et al: Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. Ann Surg 228:508-517, 1998.
25. Yeo CJ, Cameron JL, Lillemoe KD, et al: Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236:355-366, 2002.
26. Bold RJ, Charnsangavej C, Cleary KR, et al: Major vascular resection as part of pancreaticoduodenectomy for cancer: Radiologic, intraoperative, and pathologic analysis. J Gastrointest Surg 3:233-243, 1999.
27. Leach SD, Lee JE, Charnsangavej C, et al: Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg 85:611-617, 1998. 28. Neoptolemos JP, Russell RC, Bramhall S, et al: Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg 84:1370-1376, 1997.
29. Gouma DJ, van Geenen RC, van Gulik TM, et al: Rates of complications and death after pancreaticoduodenectomy: Risk factors and the impact of hospital volume. Ann Surg 232:786-795, 2000.
30. Birkmeyer JD, Siewers AE, Finlayson EV, et al: Hospital volume and surgical mortality in the United States. N Engl J Med 346:1128-1137, 2002.
31. Kotwall CA, Maxwell JG, Brinker CC, et al: National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol 9:847-854, 2002.
32. Simunovic M, To T, Theriault M, Langer B: Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ 160:643-648, 1999.
33. Birkmeyer JD, Warshaw AL, Finlayson SR, et al: Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 126:178-183, 1999.
34. Birkmeyer JD: Raising the bar for pancreaticoduodenectomy. Ann Surg Oncol 9:826-827, 2002.
35. Neoptolemos JP: Pancreatic cancer-a major health problem requiring centralization and multidisciplinary team work for improved results. Dig Liv Dis 34:692-695, 2002.
36. Sohn TA, Yeo CJ, Cameron JL, et al: Resected adenocarcinoma of the pancreas- 616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 4:567-579, 2000.
37. Pisters PWT, Hudec W, Hess KR, et al: Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 234:47-55, 2001.
38. Balcom JH, Rattner DW, Warshaw AL, et al: Ten-year experience with 733 pancreatic resections: Changing indications, older patients, and decreasing length of hospitalization. Arch Surg 136:391-398, 2001.
39. Yeo CJ: Management of complications following pancreaticoduodenectomy. Surg Clin North Am 75:913-924, 1995.
40. Spitz FR, Abbruzzese JL, Lee JE, et al: Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 15:928-937, 1997.
41. Pendurthi TK, Hoffman JP, Ross E, et al: Preoperative versus postoperative chemoradiation for patients with resected pancreatic adenocarcinoma. Am Surg 64:686-692, 1998.
42. Exocrine pancreas, in AJCC Cancer Staging Manual, pp 157-164. New York, Springer-Verlag, 2002.
43. Breslin T, Hess KR, Harbison DB, et al: Neoadjuvant chemoradiation for adenocarcinoma of the pancreas: Treatment variables and survival duration. Ann Surg Oncol 8:123-132, 2001.
44. Moertel CG, Childs DSJ, Reitemeier RJ, et al: Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 2:865-867, 1969.
45. Moertel CG, Frytak S, Hahn RG, et al: Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 48:1705-1710, 1981.
46. Kalser MH, Ellenberg SS: Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection [published erratum appears in Arch Surg 121:1045, 1986]. Arch Surg 120:899-903, 1985.
47. Bakkevold KE, Arnesjo B, Dahl O, et al: Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater-results of a controlled, prospective, randomised multicentre study. Eur J Cancer 29A:698-703, 1993.
48. Klinkenbijl JH, Jeekel J, Sahmoud T, et al: Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776-782, 1999.
49. Neoptolemos JP, Dunn JA, Stocken DD, et al: Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 358:1576- 1585, 2001.
50. Takada T, Amano H, Yasuda H, et al: Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95:1685-1695, 2002.
51. Gastrointestinal Tumor Study Group: Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 59:2006-2010, 1987.
52. Neoptolemos JP, Stocken DD, Friess H, et al: A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200-1210, 2004.
53. Gastrointestinal Tumor Study Group: Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Eng J Med 312:1465-1472, 1985.
54. Wayne JD, Abdalla EK, Wolff RA, et al: Localized adenocarcinoma of the pancreas: The rationale for preoperative chemoradiation. Oncologist 7:34-45, 2002.
55. Wolff RA, Evans DB, Crane CH, et al: Initial results of preoperative gemcitabine (GEM)-based chemoradiation for resectable pancreatic adenocarcinoma (abstract 516). Proc Am Soc Clin Oncol 21:130a, 2002.
56. Pisters PWT, Abbruzzese JL, Janjan NA, et al: Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol 16:3843-3850, 1998.
57. Pisters PW, Wolff RA, Janjan NA, et al: Preoperative paclitaxel and concurrent rapidfractionation radiation for resectable pancreatic adenocarcinoma: Toxicities, histologic response rates, and event-free outcome. J Clin Oncol 20:2537-2544, 2002.
58. Pisters PW, Hudec WA, Lee JE, et al: Preoperative chemoradiation for patients with pancreatic cancer: Toxicity of endobiliary stents. J Clin Oncol 18:860-867, 2000.
59. Crane CH, Janjan NA, Evans DB, et al: Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol 29:9-18, 2001.
60. Jain RK: Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med 7:987-989, 2001.
61. Jeekel J, Treurniet-Donker AD: Treatment perspectives in locally advanced unresectable pancreatic cancer. Br J Surg 78:1332-1334, 1991.
62. Jessup JM, Steele G Jr, Mayer RJ, et al: Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg 128:559-564, 1993.
63. Kallimanis GE, Gupta PK, al Kawas FH, et al: Endoscopic ultrasound for staging esophageal cancer, with or without dilation, is clinically important and safe. Gastrointest Endosc 41:540-546, 1995.
64. Todd KE, Gloor B, Lane JS, et al: Resection of locally advanced pancreatic cancer after downstaging with continuous-infusion 5-fluorouracil, mitomycin-C, leucovorin, and dipyridamole. J Gastrointest Surg 2:159-166, 1998.
65. Bajetta E, Di Bartolomeo M, Stani SC, et al: Chemoradiotherapy as preoperative treatment in locally advanced unresectable pancreatic cancer patients: Results of a feasibility study. Int J Radiat Oncol Biol Phys 45:285- 289, 1999.
66. Kornek GV, Schratter-Sehn A, Marczell A, et al: Treatment of unresectable, locally advanced pancreatic adenocarcinoma with combined radiochemotherapy with 5-fluorouracil, leucovorin and cisplatin. Br J Cancer 82:98- 103, 2000.
67. White R, Lee C, Anscher M, et al: Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 6:38-45, 1999.
68. Trede M, Schwall G, Saeger HD: Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 211:447-458, 1990.
69. Willett CG, Lewandrowski K, Warshaw AL, et al: Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 217:144-148, 1993.
70. Nitecki SS, Sarr MG, Colby TV, et al: Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 221:59-66, 1995.
71. Sperti C, Pasquali C, Ferronato A, et al: Median pancreatectomy for tumors of the neck and body of the pancreas [see comments]. J Am Coll Surg 190:711-716, 2000.
72. Nishimura Y, Hosotani R, Shibamoto Y, et al: External and intraoperative radiotherapy for resectable and unresectable pancreatic cancer: Analysis of survival rates and complica- tions. Int J Radiat Oncol Biol Phys 39:39-49, 1997.
73. Millikan KW, Deziel DJ, Silverstein JC, et al: Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 65:618-623, 1999.