Continuous Low-Dose GM-CSF as Salvage Therapy in Refractory Recurrent Breast or Female Genital Tract Carcinoma
Granulocyte-macrophage colony-stimulating factor (GM-CSF,sargramostim [Leukine]) is a powerful cytokine that is able to stimulatethe generation of dendritic cells. Adjuvant treatment with continuous lowdoseGM-CSF has been shown to prolong survival of stage III/IV melanomapatients. Data on continuous low-dose GM-CSF therapy in tumorsother than prostate cancer are still lacking.
Granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim [Leukine]) is a powerful cytokine that is able to stimulate the generation of dendritic cells. Adjuvant treatment with continuous lowdose GM-CSF has been shown to prolong survival of stage III/IV melanoma patients. Data on continuous low-dose GM-CSF therapy in tumors other than prostate cancer are still lacking. This pilot trial was initiated in order to evaluate the efficacy and tolerability of continuous low-dose GM-CSF as salvage in various chemotherapy-refractory carcinomas. A total of 19 patients who had failed a median of 4 prior chemotherapies were included. Their malignancies included metastatic breast cancer, recurrent ovarian carcinoma, metastatic endometrial carcinoma, and recurrent squamous cell cancer of the cervix uteri. Continuous low-dose GM-CSF was delivered subcutaneously at a daily starting dose of 125 μg. GM-CSF was increased at 25-μg increments until a maximum of 250 μg was reached or when mild leukocytosis (10–20 g/L) was achieved, providing that the relative eosinophil count did not exceed 15%. Therapy was continued until progression or refusal by the patient. Toxicity was generally mild. Only one patient was withdrawn due to grade 3 fatigue. In three additional patients, temporary dose reduction was necessary because of grade 1 injection site reactions, which recovered spontaneously. Mild to moderate leukocytosis was obvious in 10 patients. Systemic hypersensitivity- like reactions did not occur and no patient required hospitalization for other life-threatening side effects. The objective response rate was 37%: 1 complete and 6 partial responses, 4 disease stabilizations, 8 progression of disease. Median response duration was 6 months. Notably, 6 of 7 responders but only 1 of 8 patients with disease progression developed leukocytosis during therapy. Therefore, we conclude that continuous lowdose GM-CSF has substantial activity in heavily pretreated patients with either metastatic breast cancer or female genital tract cancer. Achievement of mild leukocytosis seems to be a predictor of response.
Granulocyte-macrophage colony- stimulating factor (GMCSF, sargramostim [Leukine]) has been widely used to restore bone marrow function after dose-intensive chemotherapy. In various clinical scenarios, both granulocyte colony-stimulating factor (G-CSF, filgrastim [Neupogen]) and GM-CSF have been found to effectively prevent chemotherapy- induced neutropenia. Compared to G-CSF, however, the clinical use of GM-CSF as an adjunct to antineoplastic chemotherapy has been compromised by the activation of eosinophils, which may well have been the main reason for a considerable incidence of treatment-related type I hypersensitivity-like reactions. More recently, however, GM-CSF has been recognized as a powerful cytokine playing a major role in the generation of dendritic cells from mononuclear precursors.[1,2]
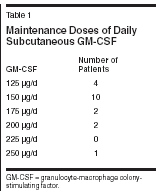
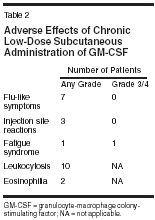
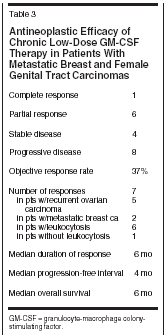
Dendritic cells are the most important antigen-presenting cells able to establish an intrinsic cellular immunologic response against autologous tumor cells. GM-CSF has therefore been used as part of cytokine cocktails for ex vivo expansion of dendritic cells from peripheral blood mononuclear cells.[2] Local or systemic administration of GM-CSF alone or in combination with interferons and interleukins also increases the dendritic cell fraction in vivo.[3,4] In metastatic melanoma, GM-CSFbased cytokine combinations have now become a routinely used immunotherapy.[ 3,5] In contrast, singleagent GM-CSF treatment is far less well established at present. However, adjuvant low-dose prolonged administration GM-CSF has significantly improved the median survival of patients with stage III and IV melanoma in a single institution trial.[5] The local and systemic toxicity of this regimen was remarkably low. Moreover, peritumoral GM-CSF injection has prolonged the survival of individuals with metastatic melanoma, an effect that cannot be explained by local effects alone.[6] At present, data on systemic GMCSF treatment in epithelial tumors other than prostate cancer are still lacking.[7,8] Nonetheless, this approach may be particularly interesting in gynecologic tumors since GM-CSF modulated activation of transforming growth factor-beta (TGF-beta) receptors has been shown to be a negative autocrine growth regulation mechanism in derivates of normal and neoplastic Mllerian epithelium.[ 9,10] Based on observations we have made in some individuals with breast or ovarian carcinoma treated with low-dose subcutaneous molgramostim, chronic rather than intervallic administration of GM-CSF may be crucial for establishing a sustained antitumoral immune response (Kurbacher, unpublished data, 2002). This open-label pilot trial was therefore initiated to investigate the activity and toxicity of continuous low-dose GM-CSF monotherapy in heavily pretreated patients suffering from either metastatic breast or female genital tract carcinomas. Patients and Methods A total of 19 patients with intensively pretreated metastatic breast cancer (n = 7), recurrent ovarian carcinoma (n = 10), recurrent endometrial carcinoma (n = 1), or recurrent squamous cell cancer of the cervix uteri (n = 1) were recruited into this pilot trial. Prior to study entry, patients had failed a median of 4 prior systemic treatments (range: 2-7). Metastatic breast cancer patients had undergone 3 to 5 (median: 5) prior therapies including anthracyclines in 6 cases and taxanes in 5. Patients with gynecologic tumors had failed a median of 3 (range: 2-7) prior cytotoxic treatments, all comprising both platinum compounds and taxanes. Patients had either bidimensionally measurable lesions or evaluable disease (elevated or rising CA 125 serum levels). Inclusion criteria were as follows: (a) Karnofsky performance status ≥ 60%, (b) absence of severe hematologic and serologic abnormalities, (c) no history of colony-stimulating factor (CSF)-related homogeneously staining regions or any other serious adverse events related to the use of colony-stimulating factors, (d) absence of serious medical disorders other than those related to advanced tumor burden, (e) estimated life expectancy ≥ 8 weeks, and (f) written informed consent. This trial was performed in accordance with the institutional ethical guidelines and the German law (Arzneimittelgesetz). GM-CSF was administered as daily subcutaneous injections. Hematologic functions were controlled every second week by whole blood cell counts with leukocyte differentiation. Therapy was monitored by biweekly tumor marker determinations and an adequate tumor imaging performed every 8 weeks (in patients with measurable disease). The starting dose of GM-CSF was 125 μg/d, which was increased at increments of 25 μg/d after every second week of treatment up to a maximum of 250 μg/d until an objective tumor response was achieved. Dose intensification was discontinued in the case of any local or systemic toxicity, a total leukocyte count > 20 g/L, or eosinophils > 15%. GM-CSF therapy was continued until there was clear evidence of primary or secondary treatment failure or the occurrence of side effects exceeding National Cancer Institute Common Toxicity Criteria (NCICTC) grade 2. Responses were classified using common Eastern Cooperative Oncology Group (ECOG) criteria (in patients with measurable disease) or Rustin criteria (in patients with evaluable disease). Overall survival was calculated from the start of therapy until death from any cause or loss to follow-up. Results All patients treated were evaluable for both toxicity and response. The maintenance dose was 125 μg/d in 4 patients, 150 μg/d in 10, 175 μg/d in 2, 200 μg/d in 2, and 250 μg/d in 1 patient, respectively (median: 150 μg/d; mean: 158 μg/d) (see Table 1). Treatmentrelated toxicities are shown in Table 2. Ten patients developed mild to moderate leukocytosis (10-20 g/L). Generally, GM-CSF treatment was well tolerated. No patient suffered from hypersensitivity-like reactions, fluid retention, edema, or impairment of cardiac function. Mild to moderate flu-like symptoms (arthralgia, myalgia, bone pain, mild to moderate febrile reactions) not exceeding NCI-CTC grade 2 were the most frequent side effects. Grade 1 injection site reactions occurred in three patients. In only one patient, GM-CSF therapy was terminated because of unresolved grade 3 fatigue requiring hospitalization. Any other severe or life-threatening complications were not observed. Detailed information concerning therapeutic efficacy can be obtained from Table 3. One complete and six partial responses accounted for an objective response rate of 37% (confidence interval [CI]: 16%-62%) with a median response duration of 6 months (range: 4-13 months). Four additional patients achieved disease stabilization of 3 to10 months. Only eight patients progressed on therapy. In summary, 11 patients (58%, CI: 34%-80%) benefited from GM-CSF treatment. It should be noted that 5 of 7 patients responding to treatment and 8 of 11 patients who benefited had recurrent ovarian carcinoma.
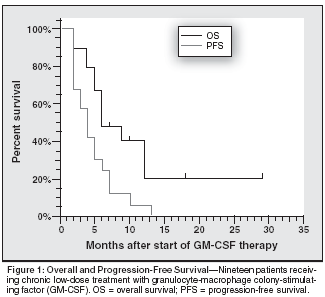
Recently, only one patient was free from progression and seven patients are still alive, resulting in a median progression-free survival of 4 months and a median overall survival of 6 months (see Figure 1). Patients benefiting from therapy had a progression- free survival of 6 months. Six of these patients are still alive, so that the median survival time in this subgroup has not yet been reached. Interestingly, 6 of 7 patients responding to GM-CSF and 9 of 11 patients benefiting from GM-CSF but only 1 of 8 patients with progression developed mild to moderate leukocytosis.
Discussion GM-CSF is a powerful cytokine that plays a crucial role in the generation of antigen-presenting cells from blood and tissue precursors both ex vivo and in vivo.[2,5] Accompanying interleukins and interferons, it is therefore an integral component of different combination regimes used for both immunotherapy of clinical malignancies and ex vivo activation of dendritic cells.[2] Recently, an increasing body of evidence exists suggesting that GM-CSF treatment is able to expand the dendritic cell population in both the peripheral blood and tumorassociated lymph nodes.[3,4] Compared to immunotherapy using ex vivo generated dendritic cells, GM-CSF given directly to patients may be a reasonable and easier to handle alternative. In advanced malignant melanoma, single-agent subcutaneous or perilesional GM-CSF has already been used successfully as systemic treatment after complete or incomplete surgical resection.[5] However, experience with this therapy in other solid tumors including breast and gynecologic malignancies is still limited. In prostate cancer, some serologic responses and disease stabilizations have been reported.[7,8] The incidence of both severe toxicities and elevation of white blood cell counts was neglected in all these trials, which did not utilize GM-CSF continuously. However, our personal experience with two patients previously treated with molgramostim has led us to conclude that continuous exposure to GMCSF may be crucial for a maintained immunologic response (Kurbacher, unpublished data, 2002). Since the balance of pro- and antitumoral effects induced by GM-CSF appears to be dose-dependent, administration of very low doses may well be another important determinant of its clinical efficacy.[11] This trial, which to our knowledge is the first of its kind in breast or female genital tract cancer, was therefore initiated to evaluate both the safety and antineoplastic efficacy of chronic low-dose subcutaneous GM-CSF. Starting with a very low dose of 125 μg per day, most patients were exposed to chronic GM-CSF at 150 to 175 μg daily. In general, the treatment was well tolerated. Only one patient experienced grade 3 toxicity (fatigue) and was thus withdrawn from the study. As could be expected from other immunotherapy trials, flu-like symptoms were frequent but never dose-limiting. Regarding the intensive pretreatment of most patients, the objective response rate of 37% with one complete response and six partial responses was exceptionally high considering that another four patients experienced durable disease stabilization. The median response duration (6 months) was long enough to indicate significant clinical utility. Interestingly, a mild to moderate increase of the whole blood count was noticed in 6 of 7 responders but in only 1 of 8 patients progressing while receiving GM-CSF. Leukocytosis may thus be a valuable surrogate marker for further clinical response to GM-CSF. At present, there is no evidence that chronic bone marrow stimulation was harmful to any of the patients. In particular, there was no evidence of impaired cardiac function in the treated population, which may be associated with higher doses of GM-CSF.[12] Although the number of patients is still too small to draw any definite conclusion, a trend suggests that patients with tumors arising from the Mllerian epithelium (specifically, ovarian carcinoma) may benefit the most from chronic GM-CSF therapy. This seems to be of particular interest since several authors have shown that GM-CSF acts as a negative autocrine growth factor in the normal and neoplastic endometrium.[9,10] It may therefore well be that GM-CSF in these tumors has a pleitropic effect by both activating the dendritic cell-mediated antitumor response and directly inducing a growth arrest by stimulating intratumoral GM-CSF receptors. Conclusion In conclusion, chronic low-dose GM-CSF was safe and highly active in this heavily pretreated population of patients. The results of our study offer a new and practical approach to immunotherapy in patients with solid tumors others than melanoma or renal cell cancer and, therefore, warrant confirmation by larger-scaled clinical trials.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.Weiss AJ, Lackman RD: A comparison of human G-CSF and human GM-CSF given concurrently with anti-cancer chemotherapy. Oncol Rep 9:945-950, 2002.
2. Chang DZ, Lomazow W, Joy Somberg C, et al: Granulocyte-macrophage colony stimulating factor: An adjuvant for cancer vaccines. Hematology 9:207-215, 2004.
3. Basak SK, Harui A, Stolina M, et al: Increased dendritic cell number and function following continuous in vivo infusion of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood 99:2869-2879, 2002.
4. Pinedo HM, Buter J, Luykx-de Bakker SA, et al: Extended neoadjuvant chemotherapy in locally advanced breast cancer combined with GM-CSF: Effect on tumour-draining lymph node dendritic cells. Eur J Cancer 39:1061-1067, 2003.
5. Spitler LE, Grossbard ML, Ernstoff MS, et al: Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol 18:1614-1621, 2000.
6. Hoeller C, Jansen B, Heere-Ress E, et al: Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol 117:371-374, 2001.
7. Dreicer R, See WA, Klein EA: Phase II trial of GM-CSF in advanced prostate cancer. Invest New Drugs 19:261-265, 2001.
8. Rini BI, Weinberg V, Bok R, et al: Prostate- specific antigen kinetics as a measure of the biologic effect of granulocyte-macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol 21:99-105, 2003.
9. Chegini N, Tang XM, Dou Q: The expression, activity and regulation of granulocyte macrophage-colony stimulating factor in human endometrial epithelial and stromal cells. Mol Hum Reprod 5:459-466, 1999.
10. Ripley D, Tang XM, Ma C, et al: The expression and action of granulocyte-macrophage colony-stimulating factor and its interaction with TGF-beta in endometrial carcinoma. Gynecol Oncol 81:301-309, 2001.
11. Li J, Bouton-Verville H, Holmes LM, et al: Inhibition or promotion of tumor growth by granulocyte-macrophage colony stimulating factor derived from engineered tumor cells is dose-dependent. Anticancer Res 24:2717- 2721, 2004.
12. Knoops S, Groeneveld AB, Kamp O, et al: Granulocyte-macrophage colony-stimulating factor (GM-CSF) decreases left ventricular function. An echocardiographic study in cancer patients. Cytokine 14:184-187, 2001.