Docetaxel and Vinorelbine Plus GM-CSF in Malignant Melanoma
Patients having locoregional or metastatic melanoma have a poorprognosis, with 50% to 100% of patients dying from the disease within5 years. Current chemotherapy regimens offer limited benefits to thesepatients, and more effective and less toxic treatments are needed. Wetherefore piloted a study of docetaxel (Taxotere), vinorelbine(Navelbine), granulocyte-macrophage colony-stimulating factor(GM-CSF, sargramostim [Leukine]), or the DVS regimen, in patientswith stage IV melanoma. Eight patients were treated after previousbiochemotherapy and two patients were given the regimen as an initialtreatment. The DVS regimen consisted of docetaxel at 40 mg/m2 IVover 1 hour, vinorelbine at 30 mg/m2 IV over 6 to 10 minutes every 14days, and GM-CSF at 250 mg/m2 SC on days 2 to 12. No grade 3 or 4toxicities were encountered. Of the 10 patients evaluable for response, 5were partial responders (50% response rate). Time to progression for the10 cases ranged from 2 to 26+ months (median: 8 months). The DVSregimen was active against advanced melanoma in both previously treatedand untreated patients. A larger study to confirm the activity of the DVSregimen for stage IV melanoma is currently under way.
Patients having locoregional or metastatic melanoma have a poor prognosis, with 50% to 100% of patients dying from the disease within 5 years. Current chemotherapy regimens offer limited benefits to these patients, and more effective and less toxic treatments are needed. We therefore piloted a study of docetaxel (Taxotere), vinorelbine (Navelbine), granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim [Leukine]), or the DVS regimen, in patients with stage IV melanoma. Eight patients were treated after previous biochemotherapy and two patients were given the regimen as an initial treatment. The DVS regimen consisted of docetaxel at 40 mg/m2 IV over 1 hour, vinorelbine at 30 mg/m2 IV over 6 to 10 minutes every 14 days, and GM-CSF at 250 mg/m2 SC on days 2 to 12. No grade 3 or 4 toxicities were encountered. Of the 10 patients evaluable for response, 5 were partial responders (50% response rate). Time to progression for the 10 cases ranged from 2 to 26+ months (median: 8 months). The DVS regimen was active against advanced melanoma in both previously treated and untreated patients. A larger study to confirm the activity of the DVS regimen for stage IV melanoma is currently under way.
FIGURE 1
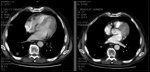
CT Scan Example of Responder
The incidence of melanoma has increased more rapidly than any other cancer, except lung cancer in women, during the past decade. There are an estimated 53,600 new cases of malignant melanoma and 7,400 deaths annually in the United States.[1] Although curable in its early stages, melanoma is the most common fatal form of skin cancer. Patients having locoregional or metastatic disease have a poor prognosis, with 50% to 100% of patients dying from the disease within 5 years.[2]
Although malignant melanoma is relatively resistant to systemic treatment, chemotherapy can induce response rates of 30% to 50% and provide palliation for some patients. Combination chemotherapy regimens that have demonstrated activity in phase II studies include the three-drug combination of cisplatin, vinblastine, and dacarbazine (CVD)[3] and the four-drug combination of cisplatin, dacarbazine, carmustine (BiCNU), and tamoxifen (CDBT).[3,4] Interferon and interleukins also produce responses in 10% to 20% of patients with metastatic melanoma.[5,6] The modest therapeutic effectiveness of chemotherapy and cytokine treatment given separately has prompted study of the two treatments given in combination, referred to as biochemotherapy.
Eton et al conducted a phase III prospective, randomized trial to compare the effects of CVD alone with sequential biochemotherapy (CVD plus interferon alfa-2b and interleukin- 2 [IL-2]) for patients with advanced melanoma. Ten percent of the patients were alive at a median of 52 months from the start of therapy. Response rates were 48% for biochemotherapy and 25% for chemotherapy (P = .001). Six patients given biochemotherapy and two given chemotherapy had complete responses. The median time to progression was 4.9 months for the biochemotherapy group and 2.4 months for the chemotherapy group (P= .008); median survival was 11.9 and 9.2 months, respectively (P= .06). However, biochemotherapy produced substantially more constitutional, hemodynamic, and myelosuppressive toxic effects in this trial.[7]
Atkins et al randomly assigned 416 patients to receive CVD, either alone or concurrent with IL-2 and interferon, and found no statistically significant differences in efficacy between the two arms. The response rate in CVD-only group was 11.4% vs 17.1% in the biochemotherapy group. The overall survival rates were 8.7 and 8.4 months, respectively. Grade IV toxicity occurred in 37% of patients on CVD vs 63% on CVD plus IL-2 and interferon. Side effects seen more frequently with biochemotherapy included hypotension, metabolic abnormalities, fatigue, nausea, hepatic dysfunction, leukopenia, thrombocytopenia, anemia, and infection. Five treatment-related deaths were reported: two in the CVD-only group and three in the biochemotherapy group.
Although biochemotherapy produced a slightly higher response rate and progression-free survival in this trial (5.3 vs 3.6 months), it was not associated with an improved quality of response or longer overall survival.[ 8] More effective and less toxic treatments are clearly needed.
Docetaxel, Vinorelbine, and GM-CSF
In preclinical studies, we observed that docetaxel (Taxotere) and vinorelbine (Navelbine) showed significant independent in vitro activity against melanoma specimens. Using the Oncotech extreme drug resistance assay to study the in vitro response of melanoma specimens, we found low drug resistance for docetaxel in 32 of 104 cases (31%) and for vinorelbine in 36 of 104 cases (35%) (personal communication, Ing-Ru Yu, 1997).
Similarly, Photiou et al reported that the taxane paclitaxel and vinorelbine act synergistically in vitro against melanoma cell lines, with both agents active in the nanomolar range at clinically achievable concentrations.[9] Using an ex vivo adenosine triphosphate (ATP)- based chemosensitivity assay, Neale et al demonstrated that 43% of vinorelbine- treated and 33% of paclitaxeltreated cutaneous melanomas showed sensitivity in the assay.[10]
The safety and clinical activity of the docetaxel/vinorelbine combination have been demonstrated in patients with locally advanced and metastatic non-small-cell lung cancer[11] and metastatic breast cancer.[12,13] Retsas et al evaluated the activity and toxicity of two sequences of paclitaxel combined with vinorelbine in disseminated malignant melanoma. Of 15 previously untreated patients, eight received vinorelbine at 30 mg/m2 (maximum dose 50 mg) first, followed 24 hours later by paclitaxel at 120 mg/m2 (maximum dose 240 mg) infused over 3 hours (VT sequence). Seven patients received the reverse sequence (TV).[14]
There were no anaphylactic episodes, and the main toxicity noted was alopecia. No significant neutropenia, emesis, or neuropathy was observed with either schedule. Three major responses were seen, all in patients who received the VT sequence. Clinically meaningful tumor regressions that did not qualify as major responses were observed in two additional patients who received the TV sequence.[14]
Granulocyte-macrophage colonystimulating factor (GM-CSF, sargramostim [Leukine]) may have adjuvant benefit in melanoma. GM-CSF is integral to the functioning of the immune system, such as activation of macrophages, an important consideration in adjuvant therapy of cancer. Activated macrophages distinguish tumor cells from normal cells and, consequently, target only the tumor cells.[15] In vitro, GM-CSF stimulates peripheral blood monocytes to become cytotoxic to human melanoma cells.[16,17] The in vivo administration of GM-CSF results in an increase in the functional capacity of monocytes, as reflected by increased cytotoxicity.[18,19] Additionally, GM-CSF, through its action on tumor infiltrating macrophages, causes the production of angiostatin, an angiogenesis inhibitor.[20,21]
In two separate melanoma models, GM-CSF was found to be the most effective of the cytokines studied for induction of long-term protective immunity.[ 22,23] GM-CSF was first studied as an adjuvant to surgery in patients with metastatic melanoma by Spitler and colleagues. In a phase II openlabel study, patients with aggressive malignant melanoma (n = 48) received GM-CSF at 125 mg/m2/d for 14 days followed by 14 days of no treatment. The cycle was repeated every 28 days for a total of 12 cycles (12 months). The survival rate at 1 year for patients receiving GM-CSF was almost double (89% vs 45%) that of historically matched controls (P < .001).[24]
The evidence of vaccine immunopotentiation by GM-CSF and the welldescribed array of T-cell targets that are candidate intermediate end points for clinical trials in melanoma, along with the data reviewed above, support more extensive evaluation of the possible immune effects of GM-CSF in this setting. Therefore, an evaluation of the activity of the DVS combination (docetaxel, vinorelbine, and GM-CSF [sargramostim]) for the treatment of patients with stage IV melanoma was undertaken.
Patients and Methods
Treatment
TABLE 1

Time to Progression of 10 Patients Treated With the DVS Regimen
A regimen of docetaxel at 40 mg/m2 IV over 1 hour, vinorelbine at 30 mg/m2 IV over 6 to10 minutes every 14 days, and GM-CSF at 250 mg/m2 SC daily on days 2 to 12 was administered to 10 patients with stage IV melanoma with measurable disease, either after biochemotherapy (n = 8) or as an initial treatment (n = 2).
Study Design
Response rates were determined by CT scans using criteria for partial response of ≥ 50% reduction in one dimension of the dominant mass that lasted > 4 weeks. Time to progression was determined from the time DVS treatment was initiated until radiologic evidence of an increase of > 25% in one dimension of the dominant mass.
Results
No grade 3 or 4 toxicities were encountered for the initial 10 cases. Response rate for 10 evaluable patients was 50%, with 5 partial responses. Time to progression for the 10 cases is shown in Table 1. The median time to progression was 8 months, which is a minor improvement over the median survival of 7.5 months reported by Atkins et al for a biochemotherapy regimen that substituted oral temozolomide (Temodar) for dacarbazine.[25] A CT scan showing evidence of a partial response in one study subject is shown in Figure 1.
Conclusions
The DVS regimen in this study was active in delaying time to progression for both previously treated and untreated patients with advanced melanoma. Toxicities demonstrated were substantially less severe than those reported for other biologic agents, such as IL-2 and interferon alfa. No grade 3/4 toxicity was noted, allowing normal daily activities. These preliminary results suggest that the docetaxel/vinorelbine combination may offer a new treatment option for patients with malignant melanoma, and that GM-CSF may be a valuable component of this regimen. A larger, phase II study to confirm the activity of the DVS regimen for stage IV melanoma, using time to progression as the primary end point, is currently under way.
Financial Disclosure:Dr. Fruehauf has received grant and/or research support from Berlex Laboratories and Pfizer. He has served as a consultant to Oncotech, and has owned stock in Oncotech.
References:
1. Jemal A, Thomas A, Murray T, et al: Cancer statistics, 2002. CA Cancer J Clin 52:23- 47, 2002.
2. Cormier J: Melanoma Update From ASCO 2004. Annual Meeting of the American Society of Clinical Oncology; June 4-8, 2004; New Orleans, LA. Available at http:// professional.cancerconsultants.com/ conference_asco_2004.aspx?id=30944. Accessed March 1, 2005.
3. Legha SS, Ring S, Papadopoulos N, et al: A prospective evaluation of a triple-drug regimen containing cisplatin, vinblastine and DTIC (CVD) for metastatic melanoma. Cancer 64:2024-2029, 1989.
4. Del Prete SA, Maurer LH, O’Donnell J: Combination chemotherapy with cisplatin, carmustine, dacarbazine and tamoxifen in metastatic melanoma. Cancer Treat Rep 69:1403-1405, 1984.
5. Lindsey KR, Rosenberg SA, Sherry RM, et al: Impact of the number of treatment courses on the clinical response of patients who receive high-dose bolus interleukin-2. J Clin Oncol 18:1954-1959, 2000.
6. Moliffe R, Hancock BW: Adjuvant therapy of malignant melanoma. Crit Rev Oncol Haematol 44:81-102, 2002.
7. Eton O, Legha SS, Bedikian AY, et al: Sequential biochemotherapy versus chemotherapy for metastatic melanoma: Results from a phase III randomized trial. J Clin Oncol 20:2045-2052, 2002.
8. Atkins MB, Lee S, Flaherty LE, et al: A prospective randomized phase III trial of concurrent biochemotherapy (BCT) with cisplatin, vinblastine, dacarbazine (CVD), IL-2 and interferon alpha-2b (IFN) versus CVD alone in patients with metastatic melanoma (E3695): An ECOG-coordinated intergroup trial (abstract 2847). Proc Am Soc Clin Oncol 22:708, 2003.
9. Photiou A, Shah P, Leong LK, et al: In vitro synergy of paclitaxel (Taxol) and vinorelbine (Navelbine) against human melanoma cell lines. Eur J Cancer 33:463- 70,1997.
10. Neale MH, Myatt NE, Khoury GG, et al: Comparison of the ex vivo chemosensitivity of uveal and cutaneous melanoma. Melanoma Res 11:601-609, 2001.
11. Sanchez JM, Balana C, Font A, et al: Phase II non-randomized study of three different sequences of docetaxel and vinorelbine in patients with advanced non-small cell lung cancer. Lung Cancer 38:309-315, 2002.
12. Rodriguez J, Calvo E, Cortes J, et al: Docetaxel plus vinorelbine as salvage chemotherapy in advanced breast cancer: A phase II study. Breast Cancer Res Treat 76:47-56, 2002.
13. Gomez-Bernal A, Cruz JJ, Garcia- Palomo A, et al: Biweekly docetaxel and vinorelbine in anthracycline-resistant metastatic breast cancer: A multicenter phase II study. Am J Clin Oncol 26:127-131, 2003.
14. Retsas S, Mohith A, Mackenzie H: Taxol and vinorelbine: A new active combination for disseminated malignant melanoma. Anticancer Drugs 7:161-165,1996.
15. Fidler IJ, Kleinerman ES: Lymphokineactivated human blood monocytes destroy tumor cells but not normal cells under cocultivation conditions. J Clin Oncol 2:937- 943, 1984.
16. Grabstein KH, Urdal DL, Tushinski RJ, et al: Induction of macrophage tumoricidal activity by granulocyte-macrophage colonystimulating factor. Science 232:506-508, 1986.
17. Thomassen MJ, Barna BP, Rankin D, et al: Differential effect of recombinant granulocyte macrophage colony-stimulating factor on human monocytes and alveolar macrophages. Cancer Res 49:4086-4089, 1989.
18. Wing EJ, Magee DM, Whiteside TL, et al: Recombinant human granulocyte/macrophage colony-stimulating factor enhances monocyte cytotoxicity and secretion of tumor necrosis factor alpha and interferon in cancer patients. Blood 73:643-646, 1989.
19. Chachoua A, Oratz R, Hoogmoed R, et al: Monocyte activation following systemic administration of granulocyte-macrophage colony-stimulating factor. J Immunother 15:217-224, 1994.
20. Kumar R, Dong Z, Fidler IJ: Differential regulation of metalloelastase activity in murine peritoneal macrophages by granulocytemacrophage colony-stimulating factor and macrophage colony-stimulating factor. J Immunol 157:5104-5111, 1996.
21. Dong Z, Kumar R, Yang X, et al: Macrophage- derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88:801-810, 1997.
22. Dranoff G, Jaffee E, Lazenby A, et al: Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 90:3539-3543, 1993.
23. Armstrong CA, Botella R, Galloway TH, et al: Antitumor effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res 56:2191-2198, 1996.
24. Spitler, LE, Grossbard ML, Ernstoff MS, et al: Adjuvant therapy of stage III and IV melanoma using granulocyte-macrophage colonystimulating factor. J Clin Oncol 18:1614-1621, 2000.
25. Atkins MB, Gollob JA, Sosman JA, et al: A phase II pilot trial of concurrent biochemotherapy with cisplatin, vinblastine, temozolomide, interleukin 2, and IFN-α2B in patients with metastatic melanoma. Clin Cancer Res 8:3075-3081, 2002.