Counseling High-Risk Women About Breast Cancer
While 90% of cancers occur as a result of factors related to lifestyle, the environment, or aging, 5% to 10% of cancers are passed down from generation to generation. Breast cancer is the most common cancer diagnosis in American women.
While 90% of cancers occur as a result of factors related to lifestyle, the environment, or aging, 5% to 10% of cancers are passed down from generation to generation. Breast cancer is the most common cancer diagnosis in American women. One out of every eight women will be diagnosed with breast cancer, translating to a 12.1% lifetime risk of breast cancer for women in the general population.[1]Hereditary breast cancer accounts for 5% to 10% of all breast cancer diagnoses, and women who carry gene mutations associated with hereditary breast cancer have a significant risk of developing both breast and ovarian cancer. Genetic tests can identify individuals at risk for hereditary breast cancer. Advanced practice nurses (APNs) play an instrumental role in identifying these women for genetic counseling. A genetic referral is of critical importance, as it can ensure that high-risk patients and their families receive potentially life-saving information about their risk levels and how they can protect themselves.
Hereditary Breast Cancer Syndromes
Hereditary Breast and Ovarian Cancer (HBOC) is caused by a deleterious germline mutation in the BRCA1 and BRCA2 genes. HBOC is the most common of all hereditary breast cancer syndromes and the most common diagnosis seen in hereditary-cancer-risk clinics. BRCA1 and/or BRCA2 are tumor-suppressor genes that all men and women have. Their normal function is to suppress cancer. Commercial molecular testing to detect genetic mutations in these two genes has been available since 1995. BRCA mutation carriers harbor a significant lifetime risk for breast cancer, ranging from 45% to 84%, and an 11%–62% lifetime risk for ovarian cancer.[2] Males who carry the BRCA2 mutation have a 7% lifetime risk for developing male breast cancer.[3] Male carriers also have an increased risk of prostate cancer, and male and female carriers have a slight increase in their risk of developing melanoma and pancreatic cancer. HBOC follows an autosomal dominant inheritance pattern. This means that only one parent (mother and/or father) needs to be a carrier of a germline deleterious mutation in either of these genes, and if this is the case, then their offspring have a 50/50 chance of inheriting the mutation. It does not mean that carriers will necessarily develop the disease, but the chance of this happening is significantly increased.
Other, rarely seen, hereditary breast cancer syndromes are Li Fraumeni syndrome and Cowden syndrome. The classic features of Li Fraumeni syndrome include early-onset breast cancer, childhood leukemia, and teenage sarcoma. Li Fraumeni syndrome results from a mutation in the p53 gene. Classical features of Cowden syndrome include breast cancer, papillary thyroid cancer, uterine cancer, trichilemmomas, lipomas, and large head circumference. Cowden syndrome is caused by a mutation in the PTEN gene. Further information about these syndromes is available at www.genetests.org
Case Studies
It is important for APNs to identify the features of hereditary breast cancer syndromes and refer individuals to a genetic counselor or nurse for further evaluation. If a hereditary cancer syndrome is identified, this will have a large impact on the medical management of patients. The following case studies highlight classic issues to consider when counseling women about hereditary breast cancer.
Case 1
“DZ” is a 31-year-old woman who was identified as a candidate for genetic counseling by her APN during her routine annual gynecologic exam. Her APN knew that DZ was potentially at high risk for breast/ovarian cancer, due to the fact that DZ’s father was recently diagnosed with breast cancer. DZ is a G2P2 (she has had two pregnancies/deliveries), has no previous history of breast biopsies, and started menarche at 11 years of age. DZ reported that her main motivation for cancer-risk counseling was to learn about her risk for breast cancer and ovarian cancer and find out if she carried a gene mutation that puts her at increased risk. She wanted the information both for herself and for her children.
At the time of her counseling visit, DZ stated that she was uncertain about how she would manage her risk if she were found to be positive for a BRCA mutation. She was hoping that she would be informed about her choices in this regard during the counseling session. DZ and her husband planned to have more children.
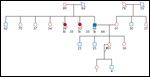
A three-generation pedigree of DZ’s family (see Figure 1) revealed her father with male breast cancer at age 66 and two paternal aunts with breast cancer at age 35 (both deceased). No one in the family had undergone genetic testing and her father was not interested in testing at this time. Testing for BRCA1 and BRCA2 was ordered.
Case 2
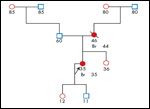
“DC” is a 30-year-old white female with a diagnosis of invasive ductal carcinoma of the left breast, with lymph node involvement. Her cancer was triple-negative (estrogen-receptor, progesterone-receptor, and HER2-receptor negative). Her breast surgeon and breast nurse navigator referred her for genetic counseling within days of her cancer diagnosis, so that a test result could be available by the time she completed her neoadjuvant chemotherapy for the breast cancer. A mastectomy of her affected breast was planned based on her current cancer diagnosis. The nurse navigator reported that DC was very nervous about her genetic counseling visit. At the time of cancer-risk counseling, DC was very clear about her desires for genetic testing and what she would do if she were found to carry a BRCA mutation. She is a G3P3 (three pregnancies/deliveries) who had no previous biopsies prior to her breast cancer diagnosis, and she experienced menarche at 10 years of age. She was not planning to have any more children. In developing a three-generation pedigree, it was noted that her mother had been diagnosed with breast cancer when she was 44 years old and had died at age 46 (see Figure 2).
Nursing Implications
Key Points
Identification of High-Risk Patients
Individuals who are at significantly high risk for cancer can be identified through cancer-predisposition testing. The ability to identify high-risk individuals who may benefit from cancer prevention and early-cancer-detection strategies can improve their length and quality of life.[4] The Oncology Nursing Society (ONS), the International Society of Nurses in Genetics (ISONG), and the National Comprehensive Cancer Network (NCCN) all have published statements recommending referral of high-risk individuals to a genetics specialist.[5–7]
A cancer genetics specialist is someone with advanced training in human genetics and cancer genetics. This may be an Advanced Practice Nurse in Genetics, a board-certified genetic counselor, or a medical geneticist. Since direct-to-consumer marketing of genetic testing is on the rise, it is important for APNs to recognize the value of genetic counseling prior to genetic testing and refer their patients accordingly. Cancer risk–assessment and counseling prior to genetic testing will educate an individual and family about the benefits and risks associated with predisposition genetic testing.[8] Key red flags for hereditary cancer syndromes are diagnosis 10 to 20 years earlier than the average age of diagnosis, multiple generations or first-degree relatives affected, an individual with two or more primary cancers, certain combinations of cancers, rare cancers, or existence in the family of a known hereditary gene mutation. The NCCN offers guidelines for referring patients to a cancer genetics specialist (see Table 1).[7]
Cancer Risk Counseling
Genetic counseling is designed to help guide patients through the cancer risk–counseling process to identify their risk for cancer (Table 2). Cancer-risk counselors also help patients who are uncertain about their family’s medical history or have concerns about other cancers, by obtaining medical records and pathology reports from the various healthcare centers at which patients and their family members have been treated. Many cancer genetics specialists work together in a multidisciplinary team that may include medical oncologists, advanced-practice nurses in genetics, board-certified genetic counselors, and Master’s-prepared licensed clinical social workers.


Special considerations for counseling high-risk womenReproductive options-BRCAmutation carriers often have concerns about bearing offspring who have a 50% chance of carrying the same BRCA mutation. Carriers should understand that BRCA mutations confer an increased risk for cancer, but not an absolute risk, and there are risk-management options both for early detection and prevention. That said, carriers might want to explore assistive reproductive technology in the form of preimplantation genetic diagnosis.[9] All carriers are offered the option for referral to a fertility specialist.[7]
Side effects of risk-reducing salpingo-oophorectomy (RRSO)-RRSO to reduce the risk of ovarian cancer is not without side effects for premenopausal women. Women may experience menopausal symptoms such as vaginal dryness, reduced interest in sex, hot flashes, sleep disturbances, and changes in sexual function. Women who are considering RRSO to reduce their high risk of ovarian cancer should be counseled prior to their surgery.[10] Women often want more information about what impact RRSO surgery may have on their sex life, and about the availability of sex counseling and the risk of coronary heart disease.[11]
Research-A benefit to referring women to genetic counseling is the option to enroll in a research study. Many community cancer genetic programs are partnering with large academic centers, to offer research studies to their patients.[12] Research will push the science forward to gain even greater understanding about BRCA mutations so that healthcare providers can further personalize care for patients, perhaps in the future enabling treatment by mutation status and location of mutations within the BRCA genes.
Legal, ethical, and social implications-During the cancer risk–counseling session, these are major points of discussion. Many patients are very concerned about the risk of discrimination and potential adverse outcomes regarding employment and health insurance, when their risk for cancer becomes part of their personal medical record. In 2008, the Genetic Information Nondiscrimination Act (GINA) was passed.[13] This federal law protects individuals with genetic mutations from health insurance and employment discrimination and advises a course of action if discrimination were to occur. Detailed information can be accessed online at www.ginahelp.org.
Case 1: Disclosure Visit
Based on DZ’s family history of male breast cancer and the early age of diagnosis in her aunt and grandmother, she met NCCN guidelines for genetic testing for BRCA1 and BRCA2 mutations.[7] Male breast cancer is a strong indicator of a BRCA mutation. In a study of 26 high-risk families with at least 1 case of male breast cancer, 77% had a BRCA2 mutation.[14] DZ was tested and was found to be a BRCA2 mutation carrier. Her husband accompanied her to the disclosure visit to discuss her test results.
Her options for managing her 85% risk for breast cancer include annual mammogram and breast MRI (magnetic resonance imaging),[15] every-6-month clinical breast examinations, and monthly breast self-exams.[7] Tamoxifen can be combined with screening to offer a 62% reduction in breast cancer risk for BRCA2 mutation carriers.[16] However, tamoxifen was not offered at this time because DZ and her husband still want to try to have more children together, and tamoxifen is contraindicated during pregnancy.
Another option for DZ would be prophylactic mastectomy with or without reconstruction, which will reduce her risk of breast cancer by more than 90%.[17] DZ was not interested in persuing this option. Also of concern was DZ’s increased risk of ovarian cancer. RRSO at age 35 or at the time of completion of childbearing reduces the risk of ovarian cancer in BRCA mutation carriers by 95%, and an added benefit to RRSO is the reduction in breast cancer risk, particularly for carriers of BRCA2 mutations.[18]
Because DZ and her husband wanted to have more children together, they decided to revisit prophylactic options for DZ when their family was complete. If DZ reached age 35 and still was not ready for prophylactic surgery, she could consider concurrent transvaginal ultrasound and serial CA-125 levels to assess her ovarian cancer risk. In addition, it is important for DZ to share her test results with her sister, who has a 1 in 2 chance of carrying the same genetic mutation. DZ’s young children will not need carrier testing until they are 18 years old.
Case 2: Disclosure Visit
Based on an early age of diagnosis and her mother’s history of breast cancer, DC met NCCN criteria for BRCA1 and BRCA2 testing.[7] DC was tested and was found to be positive for a BRCA1 mutation. A recent study found that 11% of women under 40 years of age with triple-negative breast cancer carried a BRCA1 mutation.[19]
DC’s BRCA mutation conferred a high risk of a second breast cancer and ovarian cancer. She was confident in her decision to have her opposite breast removed at the time of mastectomy of her affected breast with breast reconstruction. This will offer her a significant reduction in risk of a new breast primary. After she recovered from her breast surgery, DC underwent RRSO performed by a gynecologic oncologist. Her doctor was able to manage her surgical menopause with nonhormonal therapy.
DC utilized the book Ovarian Cancer Risk-Reducing Surgery: A Decision-Making Resource (available free of charge by emailing surgerybook@fccc.edu) to help her make her decision about surgery and to explore potential side-effects of the surgery. Of note, DC’s gynecologic oncologist ordered serial sectioning of her ovaries and did peritoneal washings because DC was a mutation carrier and at high risk for ovarian cancer.[20] DC planned to share her results with her sister and, at the appropriate time, with her young children, who all had a 50% change of carrying the same BRCA1 mutation.
The Future
On the horizon for women at high risk for breast cancer are new gene discoveries and cancer treatments based on genetic status. For example CHEK2 is a gene that when mutated does increase the risk for breast cancer.[21] However, its clinical utility is still under investigation. Testing for CHEK2 mutations is not routinely offered in the United States. Trials using PARP (poly [ADP-ribose] polymerase) inhibitors are also underway. These trials are aiming to treat women with breast cancer who are BRCA1-positive.
Summary
Due to the rapidly evolving body of research in hereditary breast cancer, APNs are challenged to maintain their knowledge base and recognize which patients should be referred to a genetics specialist. Patients look to their APNs for answers and guidance. APNs are in a position to advocate for their patients and refer them for genetic counseling. Genetic counseling offers women at high risk for breast cancer the opportunity to make informed decisions about their choices for genetic testing and how they and their family members will utilize the potentially life-saving information.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1.
American Cancer Society: Breast Cancer Facts and Figures 2009â2010. Available at
www.cancer.org
/. Accessed on August 31, 2010.
2.
Antoniou A, Pharoah PD, Narod S, et al: Average risks of breast and ovarian cancer associated with
BRCA1
or
BRCA2
mutations detected in case series unselected for family history: A combined analysis of 22 studies.
Am J Hum Genet
72(5):1117â1130, 2003.
3.
Thompson D, Easton D; Breast Cancer Linkage Consortium: Variation in cancer risks, by mutation position, in
BRCA2
mutation carriers. Am J Hum Genet 68(2):410â419, 2001.
4.
Kirk M, Lea D, Skirton H: Genomic health care: Is the future now? Nurs Health Sci 10(2):85â92, 2008.
5.
Oncology Nursing Society: Cancer predisposition genetic testing and risk assessment counseling. Available at
www.ons.org
Accessed on August 30, 2010.
6.
International Society of Nurses in Genetics: Genetic Counseling for Vulnerable Populations: The Role of Nursing. Available at
http://isong.org/ISONG_PS_genetic_counseling_vulnerable_populations.php
. Accessed on September 20, 2010.
7.
National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian. V.1.2010. Available at
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
Accessed on August 22, 2010.
8.
Lashley FR:
Essentials of Clinical Genetics in Nursing Practice
. New York, Springer Publishing, 2006.
9.
Quinn GP, Vadaparampil ST, Bower B, et al: Decisions and ethical issues among
BRCA
carriers and the use of preimplantation genetic diagnosis.
Minerva Med
100(5):371â383, 2009.
10.
Fang C, Cherry C, Devarajan K, et al: A prospective study of quality of life among women undergoing risk-reducing salpingo-oophorectomy versus gynecologic screening for ovarian cancer.
Gynecol Oncol
112(3):594â600, 2009.
11
. Campfield Bonadies D, Moyer A, Matloff ET: What I wish I’d known before surgery:
BRCA
carriers’ perspectives after bilateral salipingo-oophorectomy.
Fam Cancer
, Sept 18, 2010. [Epub ahead of print]
12
. LeGrazie B, Masny A: Establishment of a cancer genetic risk assessment program, in Calzone K, Masny A, Jenkins J (eds): Genetics and Genomics in Oncology Nursing Practice.
Oncology Nursing Society
, Pittsburgh, 2010.
13.
H.R. 493: Genetic Information Nondiscrimination Act of 2008 (GINA). Public Law No. 110-233. Available at:
http://www.govtrack.us/congress/bill.xpd?bill=h110-493
. Accessed on January 9, 2010.
14
. Ford D, Easton DF, Stratton M, et al: Genetic heterogeneity and penetrance analysis of the
BRCA1
and
BRCA2
genes in breast cancer families. The Breast Cancer Linkage Consortium.
Am J Hum Gene
t 62(3):676â689, 1998.
15.
Lehman, CD, Smith, RA: The role of MRI in breast cancer screening.
J Natl Compr Canc Netw
7(10):1109â1115, 2009.
16.
King MC, Wieand S, Hale K, et al: Tamoxifen and breast cancer incidence among women with inherited mutations in
BRCA1
and
BRCA2
: National Surgical Adjuvant Breast and Bowel Project (NSABP- P1) Breast Cancer Prevention Trial.
JAMA
286:2251â2256, 2001.
17.
Hartmann LC, Sellers TA, Schaid DJ, et al: Efficacy of bilateral prophylactic mastectomy in
BRCA1
and
BRCA2
gene mutation carriers.
J Natl Cancer Inst
93(21):1633â1637, 2001.
18.
Rebbeck TR, Lynch HT, Neuhausen SL, et al: Prophylactic oophorectomy in carriers of
BRCA1
or
BRCA2
mutations.
N Engl J Med
346(21):1616â1622, 2002.
19.
Young SR, Pilarski RT, Donenberg T, et al: The prevalence of
BRCA1
mutations among young women with triple-negative breast cancer.
BMC Cancer
9:86, 2009.
20.
Domchek SM, Friebel TM, Garber JE, et al: Occult ovarian cancers identified at risk-reducing salpingo-oophorectomy in a prospective cohort of
BRCA1
/2 mutation carriers.
Breast Cancer Res Treat
124(1):195â203, 2010.
21.
Narod SA: Testing for CHEK2 in the cancer genetics clinic: Ready for prime time?
Clin Genet
78(1):1â7, 2010.