Current Management of Adult T-Cell Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma (ATL) is defined as a histologically or cytologically proven peripheral T-cell malignancy associated with a retrovirus, human T-cell lymphotropic virus type I (HTLV-1).[1] Southwestern Japan is the district with the highest prevalence of HTLV-1 infection and the highest incidence of ATL in the world. A high prevalence of HTLV-1 infection is also found in the Caribbean islands, tropical Africa, South America, and northern Oceania.
When oncologists diagnose patients suspected of lymphoid malignancy, it is important to consider the possibility of adult T-cell leukemia/lymphoma (ATL) with a routine check for serum human T-cell lymphotropic virus type 1 (HTLV-1) antibody. The following points are essential for the diagnosis of ATL: (1) cytologically or histologically proven peripheral T-cell malignancy, and (2) positivity for anti-HTLV-1 antibody. When a patient is diagnosed with ATL, it is important to make an accurate diagnosis of clinical subtype in order to make appropriate treatment decisions. For patients with smoldering or chronic type ATL, close observation with careful monitoring for opportunistic infections is recommended. For patients with the acute or lymphoma type requiring therapy, enrollment in a clinical trial is recommended. When there is no active trial or the patient is ineligible for a trial, we recommend intensive chemotherapy used for aggressive non-Hodgkin lymphoma such as the LSG15 regimen (VCAP-AMP-VECP) based on a recent phase III study. Because most patients with ATL are not curable with current chemotherapy regimens, it is reasonable to consider the applicability of allogeneic stem cell transplantation in patients who show responses to chemotherapy. For relapsed or refractory patients, enrollment in a new-agent trial should be considered in addition to stem cell transplantation.
Adult T-cell leukemia/lymphoma (ATL) is defined as a histologically or cytologically proven peripheral T-cell malignancy associated with a retrovirus, human T-cell lymphotropic virus type I (HTLV-1).[1] Southwestern Japan is the district with the highest prevalence of HTLV-1 infection and the highest incidence of ATL in the world. A high prevalence of HTLV-1 infection is also found in the Caribbean islands, tropical Africa, South America, and northern Oceania.
It is estimated that approximately 1.2 million HTLV-1–infected individuals reside in Japan, and the annual incidence of ATL is estimated to be approximately 700 in Japan. The annual rate of ATL development among HTLV-1 carriers older than 40 years is estimated at 1.5 per 1,000 in males and 0.5 per 1,000 in females. The cumulative risk of the development of ATL among HTLV-1 carriers is estimated to be 2.5% during a 70-year lifespan. In a national survey in Japan, the mean age of patients with ATL was 57.6 years. Patients in areas outside Japan are somewhat younger, with an overall mean age in the mid-40s. There is a marked increase in HTLV-1 prevalence with age until 70 years. A major reason for the increase in seroprevalence with age appears to be the decreasing prevalence of HTLV-1 in the population with time.
Potential Strategies to Eliminate ATL
HTLV-1 is transmitted via at least three routes: (1) mother-to-child transmission, mainly by breast-feeding; (2) sexual transmission, commonly from males to females; and (3) blood-borne transmission. The overall infection rate of HTLV-1 in children by seropositive mothers is estimated to be 10% to 30%.
Several intervention trials have been conducted in Japan. The seropositive mothers were advised to refrain from breast-feeding.[1] To prevent HTLV-1 transmission through blood transfusions, serologic screening for HTLV-1 in all blood donors has been conducted in Japan since 1986. Since then, no episodes of seroconversion in transfusion recipients have been recognized.
Clinical Characteristics
Typically, the neoplastic cells in the peripheral blood are markedly polylobated and have been termed flower cells,[2] although the cytologic spectrum of ATL is diverse. These cytologic features are most evident in the acute type of ATL. Lymph nodes typically show diffuse architectural effacement. Small pleomorphic lymphoid cells may predominate or may be admixed with larger cells. The transformed cells may have more pleomorphic nuclear features. Giant cells with convoluted or cerebriform nuclear contours may be present.
Skin involvement is seen in more than 50% of patients. The skin lesions are clinically and histologically diverse, and may mimic inflammatory disorders in some patients. Other frequent sites of involvement include lung and cerebrospinal fluid. The pulmonary infiltrates are generally patchy and interstitial, without formation of tumor nodules. Involvement of the central nervous system is usually manifested as meningeal infiltration without parenchymal lesions.
Immunophenotype
The neoplastic cells are CD4-positive (CD4+) T cells that strongly express the interleukin-2 receptor (IL-2R) alpha subunit, CD25. In addition, CD3 and other mature T-cell antigens (CD2, CD5) are usually expressed. Recent studies have suggested that the cells of ATL may be the equivalent of Treg cells.[3]
Pathogenesis
The onset of ATL is preceded by a long period of latency, frequently lasting longer than 4 decades. In addition, only up to 5% of all individuals infected with HTLV-1 develop ATL. The age-specific occurrence of ATL suggested a multistep carcinogenesis model.
Initial steps depend on viral gene products, among which a viral regulatory protein, Tax, plays a pivotal role. Tax was shown to be the major viral protein with oncogenic potential.[4] Tax also exerts its pleiotropic functions through direct interaction with numerous cellular proteins,[5] resulting in a unique phenotype of the infected cells. Antisense transcripts of the HTLV-1 provirus were reported.[6] The transcript can encode a novel basic leucine zipper protein, named HBZ, which interacts with CREB2 and c-Jun and suppresses the activity of Tax and activator protein-1, respectively. Several isoforms of HBZ transcripts were reported to be steadily expressed in HTLV-1–infected cells and ATL cells. The functions of these transcripts and putative protein in the context of cellular transformation are now under investigation.[7,8]
Clinical Subtypes of Disease
Based on a nationwide survey of 854 patients with ATL in Japan, the Lymphoma Study Group (LSG) of the Japan Clinical Oncology Group (JCOG) proposed diagnostic criteria for the four clinical subtypes.[2] The leukemic subtypes include the acute type, with a rapidly progressive course and most of the characteristic features of ATL-generalized lymphadenopathy, hepatosplenomegaly, skin involvement, hypercalcemia, and organ infiltration. The symptoms and signs include abdominal pain, diarrhea, ascites, pleural effusion, cough, sputum, and chest x-ray abnormalities. The smoldering type shows an indolent course (expected median survival > 5 years) and 5% or more of leukemic cells in the peripheral blood but may also include skin involvement. The chronic type, with absolute lymphocytosis (≥ 4 × 109/L) frequently showing flower cell morphology, is occasionally associated with skin involvement and lymphadenopathy, and also shows a relatively indolent course (expected median survival = 24.3 months). The lymphoma type is comprised of patients presenting with the manifestations of lymphoma without leukemic cells. In ATL, the clinical subtype is more important than the Ann Arbor stage for predicting prognosis and deciding treatment in each patient.
Clinical Aspects of Disease
ATL-particularly the aggressive forms (acute and lymphoma types)-has been found to infiltrate the stomach in 29% and the intestine in 25% of patients at autopsy. Patients with ATL experience a variety of abdominal symptoms, such as nausea, vomiting, abdominal fullness, and diarrhea, which may be attributable to the infiltration by neoplastic cells, but because of the associated immunodeficiency, opportunistic infections such as Strongyloidiasis may complicate the course.
Hepatic involvement may be found in 31% of patients with the acute or lymphoma type and is not infrequently manifested by jaundice (30%) and hepatic enzyme elevations (53%). Pulmonary complications in 18% of patients are due to leukemic infiltration in one-half of the patients and to infections with a variety of bacterial and opportunistic organisms in the remaining one-half.[2] Central nervous system involvement, mostly leptomeningeal involvement, occurs in approximately 10% of patients with ATL. The initial symptoms include muscle weakness, altered mental status, paresthesia, headache, and urinary incontinence, which are occasionally difficult to differentiate from those caused by the HTLV-1–associated neurologic disorder, tropical spastic paraparesis (TSP)/HTLV-1-associated myelopathy (HAM).
Approximately 26% of 854 Japanese patients with ATL had active infections at diagnosis.[2] The incidence was highest in the chronic and smoldering types (36%) and lower in the acute (27%) and lymphoma types (11%). The encountered infections were bacterial in 43%, fungal in 31%, protozoal in 18%, and viral in 8% of patients. The immunodeficiency at presentation in ATL patients can be exacerbated by cytotoxic chemotherapy.
Laboratory Findings
Laboratory findings also depend on the clinical subtype.[2] Leukocytosis is found in patients with the acute or chronic type at presentation, exhibiting characteristic atypical lymphoid cells with marked polymorphic nuclei or so-called flower cells. Most patients (83%) with the acute or lymphoma type have elevated serum lactate dehydrogenase (LDH) levels. The most striking laboratory finding in patients with ATL is hypercalcemia, which was found in 32%.[2]
Prognostic Factors
In ATL, age (≥ 40 years), poor performance status (≥ 2), high LDH, hypercalcemia, and four or more involved lesions were reported to be unfavorable prognostic factors.[9,10] For patients with the chronic type, the major prognostic factors are serum LDH, albumin, and blood urea nitrogen (BUN). Patients with the chronic type and normal values for these three factors have a favorable prognosis similar to the smoldering type. In contrast, patients with the unfavorable chronic type who have an abnormal value in at least one of these three factors would be candidates for cytotoxic chemotherapy.
Treatment
It is recommended that patients with the favorable chronic or smoldering type of ATL be carefully monitored for infectious complications and signs of disease progression to the acute or lymphoma type.
Without treatment, most previously untreated patients with aggressive forms of the disease (acute or lymphoma type) die within weeks or months of the diagnosis. Most patients with the smoldering type live well without chemotherapy for a considerable time (expected median survival > 5 years).
Approximately two-thirds of patients with chronic-type disease die within approximately 2.5 years from diagnosis. Patients with the lymphoma type have unfavorable prognoses, with a median survival of 10.2 months. The most aggressive form of ATL is the acute type, associated with a median survival of 6.2 months. The projected 4-year survival rates of patients with the lymphoma and acute types are only 5%.
The clinical subtype clearly determines the prognosis of each patient with ATL.[2] In patients with smoldering- or chronic-type disease, the expected time to evolution to the acute or lymphoma type (and the incidence of that conversion) remain unclear, partly due to the lack of prospective studies. However, in a recent long-term follow-up study on 90 patients with indolent ATL (65 chronic type and 25 smoldering type) at a single institution, the median survival was 4.5 years, and the projected 5-, 10-, and 15-year survival rates were 47%, 23%, and 13%, respectively.[11]
Clinical Trials in Japan
TABLE 1

Results of Important Clinical Trials of Untreated Patients With Adult T-cell Leukemia/Lymphoma
Since 1978, chemotherapy trials have been consecutively conducted for newly diagnosed patients with ATL by JCOG’s Lymphoma Study Group (Table 1).[12-17] Between 1981 and 1983, JCOG conducted a phase III trial (JCOG8101) to evaluate LSG1-VEPA (vincristine, cyclophosphamide, prednisone, and doxorubicin) vs LSG2-VEPA-M (VEPA plus methotrexate) for advanced non-Hodgkin lymphoma (NHL), including ATL.[11,12] The complete response (CR) rate of LSG2-VEPA-M for ATL (37%) was higher than that of LSG1-VEPA (17%; P = .09). However, the CR rate was significantly lower for ATL than for B-cell NHL and peripheral T-cell lymphoma (PTCL) other than ATL (P < .001). The median survival of the 54 patients with ATL was 6 months, and the estimated 4-year survival rate was 8%.
In 1987, JCOG initiated a phase II study (JCOG8701) of a multiagent combination chemotherapy called LSG4 as a treatment for advanced aggressive NHL (including ATL). LSG4 consisted of three different regimens: (1) VEPA-B (VEPA plus bleomycin), (2) M-FEPA (methotrexate, vindesine, cyclophosphamide, prednisone, and doxorubicin), and (3) VEPP-B, (vincristine, etoposide, procarbazine [Matulane], prednisone, and bleomycin).[14] The CR rate for ATL patients was improved from 28% (JCOG8101) to 43% (JCOG8701); however, the CR rate was significantly lower in ATL than in B-cell NHL and PTCL (P < .01). Patients with ATL still showed a poor prognosis, with a median survival of 8 months and a 4-year survival rate of 12%.
The disappointing results with conventional chemotherapies have led to the search for new active agents. Multicenter phase I and II studies of pentostatin (2´-deoxycoformycin) were conducted against ATL in Japan.[11,18] The phase II study revealed a response rate of 32% (10 of 31) in relapsed or refractory ATL.
These encouraging results prompted the investigators to conduct a phase II trial (JCOG9109; LSG11) with a pentostatin-containing combination as the initial chemotherapy.[15] Patients with aggressive ATL-ie, of the acute, lymphoma, or unfavorable chronic type-were eligible for this study. Unfavorable chronic-type ATL, defined as having at least 1 of 3 unfavorable prognostic factors (low serum albumin level, high LDH level, or high BUN), has an unfavorable prognosis similar to that for acute- and lymphoma-type ATL.[10] A total of 62 untreated patients with aggressive ATL (34 acute, 21 lymphoma, and 7 unfavorable chronic type) were enrolled. A regimen of vincristine, doxorubicin, etoposide, prednisone, and pentostatin was administered every 28 days for 10 cycles. Among the 61 patients evaluable for toxicity, four patients (7%) died of infections. Among the 60 eligible patients, there were 17 CRs (28%) and 14 partial responses (PRs) (overall response rate [ORR] = 52%). The median survival was 7.4 months, and the estimated 2-year survival rate was 17%. The prognosis in patients with ATL remained poor, even though they were treated with a pentostatin-containing combination chemotherapy.
In 1994, JCOG initiated a phase II trial (JCOG9303; LSG15) of an eight-drug regimen consisting of vincristine, cyclophosphamide, doxorubicin, prednisone, ranimustine, vindesine, etoposide, and carboplatin for untreated ATL.[16] Dose intensification was attempted with the prophylactic use of granulocyte colony-stimulating factor (G-CSF, Neupogen). In addition, non–cross-resistant agents such as ranimustine and carboplatin were incorporated. Ninety-six previously untreated patients with aggressive ATL were enrolled: 58 acute, 28 lymphoma, and 10 unfavorable chronic types. Approximately 81% of the 93 eligible patients responded (75/93), with 33 patients obtaining a CR (35%). The overall survival of 93 eligible patients at 2 years was estimated to be 31%, with a median survival of 13 months. Grade 4 neutropenia and thrombocytopenia were observed in 65% and 53% of the patients, respectively, whereas grade 4 nonhematologic toxicity was observed in only one patient. The investigators concluded that the LSG15 protocol is feasible, with mild nonhematologic toxicity, and may have improved the clinical outcome of patients with ATL.
FIGURE 1

Kaplan-Meier Estimate of Overall Survival for All Randomly Assigned Patients in JCOG9801
To confirm whether the LSG15 regimen is a new standard for the treatment of aggressive ATL, JCOG conducted a phase III trial comparing LSG15 with biweekly CHOP (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], and prednisone).[17] Untreated patients with aggressive ATL were assigned to receive either six courses of LSG15 every 4 weeks or eight courses of biweekly CHOP. Both treatments were supported with G-CSF and intrathecal prophylaxis. The primary endpoint was overall survival. A total of 118 patients were enrolled. The CR rate was higher in the LSG15 arm than in the biweekly CHOP arm (40% vs 25%, respectively; P = .020). As illustrated in Figure 1, overall survival at 3 years was 24% in the LSG15 arm and 13% in the biweekly CHOP arm (P =.085, two-sided P=.169). A Cox regression analysis with performance status (PS 0 vs 1 vs 2–4) as stratum for baseline hazard functions was performed to evaluate the effect on overall survival of the factors of age, B-symptoms, subtypes of ATL, LDH, BUN, bulky mass, and treatment arms. According to this analysis, the hazard ratio and P value for the treatment arms were 0.62 (95% confidence interval, 0.38 to 1.01) and P = .028 (two-sided P = .056), respectively. The difference between the crude analysis and this result was because of unbalanced prognostic factors, such as PS 0 vs 1, and the presence or absence of bulky lesions between the treatment arms. Progression-free survival rate at 1 year was 28% in the LSG15 arm compared with 16% in the biweekly CHOP arm (P = .100, two-sided P = .200). Three toxic deaths occurred in the LSG15 arm. The longer survival at 3 years and higher CR rate with LSG15 compared with biweekly CHOP suggest that LSG15 is a more effective regimen at the expense of higher toxicities, providing the basis for future investigations in the treatment of ATL.[17] The recommended treatment algorithm for ATL is shown in Figure 2.
Development of New Agents for ATL
FIGURE 2
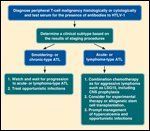
Approach to the Patient With Adult T-Cell Leukemia/Lymphoma
In 1995, Gill et al in the United States reported that 11 of 19 patients with acute or lymphomatous ATL achieved major responses (five CRs and six PRs) to the combination therapy of interferon-alpha and zidovudine.[19] The therapeutic efficacy of this combination was also observed in a French study, where major objective responses were obtained in all five patients with ATL.[20] However, the median survival of untreated patients with ATL in the US study was rather short (4.8 months) compared with those in the JCOG studies (7–13 months).[19,21] Furthermore, the CR rate in untreated patients (25%, 3/12) was not superior to the CR rates seen in JCOG8701, 9109, and 9303 (28%–42%).
Recently, the results of a meta-analysis on the use of interferon-alpha and zidovudine for ATL were reported.[1,22] A total of 100 patients received interferon-alpha and zidovudine as initial treatments. The ORR was 66%, with a 43% CR rate. In this retrospective analysis, the median survival time was 24 months and the 5 year survival rate was 50% for first-line interferon-alpha and zidovudine, vs 7 months and 20% for 84 patients who received first-line chemotherapy. The median survival of patients with acute-type ATL treated with first-line interferon-alpha/zidovudine and chemotherapy were 12 and 9 months, respectively. Patients with lymphoma-type ATL did not benefit from this combination. In addition, first-line interferon-alpha/zidovudine therapy in chronic- and smoldering-type ATL resulted in a 100% survival rate at a median follow-up of 5 years. The results for interferon-alpha and zidovudine in indolent ATL appear to be promising, although the possibility of selection bias cannot be excluded.
Because most ATL cells express the alpha-chain of IL-2R (CD25), Waldmann et al have treated patients with ATL using monoclonal antibodies to CD25.[23] Six (32%) of 19 patients who were treated with anti-Tac showed objective responses lasting from 9 weeks to longer than 3 years. One of the impediments to this approach is that a quantity of soluble IL-2R is shed by the tumor cells into the circulation. Another strategy using IL-2R is conjugation with an immunotoxin (Pseudomonas exotoxin) or radioisotope (yttrium-90). Waldmann et al developed a stable conjugate of anti-Tac with yttrium-90.[24] Among the 16 patients with ATL who received 5- to 15-mCi doses, 9 (56%) showed objective responses. The response duration was longer than that with unconjugated anti-Tac antibody.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Arsenic trioxide (Trisenox)
Bleomycine
Bortezomib (Velcade)
Carboplatin
Cyclophosphamide
Doxorubicin
Etoposide
Forodesine
Granulocyte colony-stimulating
factor (G-CSF, Neupogen)
Interferon-alpha
Lenalidomide (Revlimid)
Methotrexate
Panobinostat
Pentostatin
Pralatrexate (Folotyn)
Prednisone
Procarbazine (Matulane)
Ranimustine
Romidepsin
Vincristine
Vindesine
Vorinostat (Zolinza)
Zidovudine
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Monoclonal antibodies against molecules other than CD25, such as CD2, CD52, and chemokine receptor 4 (CCR4), appear to be promising in recent clinical trials. Ishida and coworkers conducted immunostaining analysis for CCR4 expression in 103 patients with ATL. Of the 103 cases, 91 (88%) were positive for CCR4. Multivariate analysis revealed that CCR4 expression was a significant unfavorable prognostic factor (P < .05).[25] Researchers subsequently developed a humanized anti-CCR4 monoclonal antibody, KW-0761, the Fc region of which is defucosylated to enhance antibody-dependent cell-mediated cytotoxicity. A phase I study of KW-0761 in relapsed CCR4-positive T-cell malignancy including ATL was conducted in Japan.[26] Patients received 4 weekly intravenous infusions of KW-0761 at 0.01, 0.1, 0.5, and 1.0 mg/kg. A total of 13 patients (11 ATL, 1 PTCL-unspecified, 1 mycosis fungoides) received KW-0761, which was well tolerated without any dose-limiting toxicity. The response rate for the 13 enrolled patients was 31%, including 2 CRs and 2 PRs. The investigators concluded that KW-0761 was tolerable across a wide dosage range and had clinical activity in relapsed CCR4-positive ATL or PTCL. Based on these encouraging results, a phase II study of KW-0761 for ATL was initiated in Japan.
Several new agents against ATL are now under investigation. One of the promising targeted therapies for ATL is the combination of arsenic trioxide (Trisenox) and interferon-alpha, exhibiting clinical efficacy in relapsed/refractory ATL patients.[27] Recently, results were reported from a phase II study of the combination of arsenic, interferon-alpha, and zidovudine in 10 newly diagnosed chronic ATL patients.[28] A 100% ORR was observed, including 9 CRs with no relapse at the time of reporting.
Histone deacetylase inhibitors such as vorinostat (Zolinza), romidepsin, and panobinostat are promising in preclinical and/or clinical studies. Pralatrexate (Folotyn), a novel antifolate, and forodesine, a purine nucleotide phosphorylase inhibitor, are potential new agents with potent preclinical activity in T-cell malignancies including ATL.[29] Other potential therapies for ATL under investigation include a proteasome inhibitor, bortezomib (Velcade),[30] and lenalidomide (Revlimid).
Allogeneic Stem Cell Transplantation
Fukushima et al retrospectively analyzed 40 patients who underwent allogeneic stem cell transplantation (SCT) for acute and lymphoma types of ATL in Japan.[31] The median age of patients was 44 years (range: 28–53), and 28 patients (70%) had achieved objective responses to prior chemotherapies. All evaluable patients achieved CR after allogeneic SCT, and the median survival was 9.6 months. The estimated 3-year overall and disease-free survival rates were 45% and 34%, respectively. Among 10 patients with relapsed ATL after allogeneic SCT, 5 achieved CR again, 3 by the reduction or cessation of immunosuppressive agents, which suggested a graft-vs-ATL effect. Although these results indicate that allogeneic SCT may be a promising treatment of ATL, its exact role in the treatment of ATL remains unclear. To evaluate the efficacy of allogeneic SCT more accurately, a prospective multicenter phase II study of LSG15 chemotherapy followed by allogeneic SCT will be initiated in Japan.
In addition, Okamura and associates reported the results of a multicenter feasibility study of reduced-intensity allogeneic SCT (RIST) against ATL.[32] Sixteen patients, all over 50 years of age, underwent RIST from human leukocyte antigen (HLA)-matched sibling donors. The regimen-related toxicities and nonhematologic toxicities were acceptable. Three patients who had a relapse subsequently responded to a rapid discontinuation of the immunosuppressive agent. RIST was judged to be a feasible treatment for ATL. Subsequent multicenter trials of RIST are being conducted in Japan.
Conclusion
In summary, although the majority of patients with ATL are still incurable with current treatment modalities, it is expected that investigations of novel agents and SCT would improve their outcomes in the near future.
This article is part of a Special Series on Peripheral T-cell Lymphomas, guest edited by James O. Armitage, MD, Joe Shapiro Professor of Medicine and Professor and Chair of Oncology, University of Nebraska Medical School, Omaha.
Financial Disclosure: The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Tsukasaki K, Hermine O, Bazarbachi A, et al: Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: A proposal from an international consensus meeting. J Clin Oncol 27:453-459, 2009.
2. Shimoyama M: Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma: A report from the Lymphoma Study Group (1984–87). Br J Haematol 79:428–437, 1991.
3. Roncador G, Garcia JF, Garcia JF, et al: FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia 19:2247-2253, 2005.
4. Grassmann R, Aboud M, Jeang KT: Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 24:5976-5985, 2005.
5. Yoshida M: Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 24:5931–5937, 2005.
6. Gaudray G, Gachon F, Basbous J, et al: The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZip transcription factor that down-regulates viral transcription. J Virol 76:12813–12822, 2002.
7. Satou Y, Yasunaga J, Yoshida M, et al: HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA 103:720-725, 2006.
8. Mesnard JM, Barbeau B, Devaux C: HBZ, a new important player in the mystery of adult T-cell leukemia. Blood 108:3979-3982, 2006.
9. Lymphoma Study Group: Major prognostic factors of patients with adult T-cell leukemia-lymphoma: A cooperative study. Leuk Res 15:81-90, 1991.
10. Shimoyama M: Chemotherapy of ATL, in Takatsuki K (ed): Adult T-cell leukaemia, pp 221-237. Oxford, Oxford University Press, 1994.
11. Takasaki Y, Tsukasaki K, Iwanaga M, et al: A long-term study on indolent adult T-cell leukemia/lymphoma (ATLL) (abstract 3575). Blood 110(11), 2007.
12. Shimoyama M, Ota K, Kikuchi M, et al: Chemotherapeutic results and prognostic factors of patients with advanced non-Hodgkin’s lymphoma treated with VEPA or VEPA-M. J Clin Oncol 6:128-141, 1988.
13. Shimoyama M, Ota K, Kikuchi M, et al: Major prognostic factors of adult patients with advanced T-cell lymphoma/leukemia. J Clin Oncol 6:1088-1097, 1988.
14. Tobinai K, Shimoyama M, Minato K, et al: Japan Clinical Oncology Group phase II trial of second-generation “LSG4 protocol” in aggressive T- and B-lymphoma: A new predictive model for T- and B-lymphoma (abstract). Proc Am Soc Clin Oncol 13:378a, 1994.
15. Tsukasaki K, Tobinai K, Shimoyama M, et al: Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group study (JCOG9109). Int J Hematol 77:164-170, 2003.
16. Yamada Y, Tomonaga M, Fukuda H, et al: A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukemia-lymphoma (ATL): Japan Clinical Oncology Group (JCOG) Study 9303. Br J Haematol 113:375-382, 2001.
17. Tsukasaki K, Utsunomiya A, Fukuda H, et al: VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 25:5458-5564, 2007.
18. Tobinai K, Shimoyama M, Inoue S, et al: Phase I study of YK-176 (2’-deoxycoformycin) in patients with adult T-cell leukemia-lymphoma. Jpn J Clin Oncol 22:164-171, 1992.
19. Gill PS, Harrington W, Kaplan MH, et al: Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 332:1744-1748, 1995.
20. Hermine O, Blouscary D, Gessain A, et al: Treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med 332:1749-1751, 1995.
21. Tobinai K, Kobayashi Y, Shimoyama M, et al: Interferon alfa and zidovudine in adult T-cell leukemia-lymphoma (correspondence). N Engl J Med 333:1285-1286, 1995.
22. Bazarbachi A, Panelatti G, Ramos JC, et al: A worldwide meta-analysis on the use of zidovudine and interferon-alpha for the treatment of adult T-cell leukemia/lymphoma (abstract 2049). Blood 110:610a-611a, 2007.
23. Waldmann TA: Multichain interleukin-2 receptor: A target for immunotherapy in lymphoma. J Natl Cancer Inst 81:914-923, 1989.
24. Waldmann TA, White JD, Carrasquillo JA, et al: Radioimmunotherapy of interleukin-2Ra-expressing adult T-cell leukemia with yttrium-90-labeled anti-Tac. Blood 86:4063-4075, 1995.
25. Ishida T, Utsunomiya A, Iida S, et al: Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin Cancer Res 9:3625-3634, 2003.
26. Yamamoto K, Tobinai K, Utsunomiya A, et al: Phase I study of KW-0761, a defucosylated anti-CCR4 antibody, in relapsed patients (pts) with adult T-cell leukemia-lymphoma (ATL) or peripheral T-cell lymphoma (PTCL): Updated results (abstract 1007). Blood 112(11), 2008.
27. Hermine O, Dombret H, Poupon J, et al: Phase II trial of arsenic trioxide (As2O3) and combination of alpha interferon (INF) and As2O3 in patients with relapsed/refractory adult T cell leukemia (ATL). Hematol J 5:130-134, 2004.
28. Kchour G, Tarhini M, Kooshyar M-M, et al: Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood 113:6528-6532, 2009.
29. O’Connor OA, Hamlin PA, Portlock C, et al: Pralatrexate, a novel class of antifol with high affinity for the reduced folate carrier-type 1, produces marked complete and durable remissions in a diversity of chemotherapy refractory cases of T-cell lymphoma. Br J Haematol 139:425-428, 2007.
30. Satou Y, Nosaka K, Koya Y, et al: Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia 18:1357-1363, 2004.
31. Fukushima T, Miyazaki Y, Honda S, et al: Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 19:829-834, 2005.
32. Okamura J, Utsunomiya A, Tanosaki R, et al: Allogeneic stem-cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T-cell leukemia/lymphoma. Blood 105:4143-4145, 2005.
Targeted Therapy First Strategy Reduces Need for Chemotherapy in Newly Diagnosed LBCL
December 7th 2025Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone may allow 57% of patients with newly diagnosed LBCL to receive less than the standard number of chemotherapy cycles without compromising curative potential.