Recurrent Urothelial Carcinoma With Pulmonary Metastasis
A 56-year-old woman was referred to our institution for a left nephroureterectomy after the diagnoses of a nonfunctioning left kidney and noninvasive papillary urothelial carcinoma of the distal left ureter (Ta grade 1). Following the procedure, surveillance cystoscopy and computed tomography (CT) scan of the abdomen and pelvis demonstrated a large bladder tumor with pan-urothelial extension.
SECOND OPINION
Multidisciplinary Consultations on Challenging Cases
The University of Colorado Denver School of Medicine faculty holds weekly second opinion conferences focusing on cancer cases that represent most major cancer sites. Patients seen for second opinions are evaluated by an oncologic specialist. Their history, pathology, and radiographs are reviewed during the multidisciplinary conference, and then specific recommendations are made. These cases are usually challenging, and these conferences provide an outstanding educational opportunity for staff, fellows, and residents in training.
The second opinion conferences include actual cases from genitourinary, lung, melanoma, breast, neurosurgery, gastrointestinal, and medical oncology. On an occasional basis,
ONCOLOGY
will publish the more interesting case discussions and the resultant recommendations. We would appreciate your feedback; please contact us at
second.opinion@uchsc.edu
.
E. David Crawford, MD
Al Barqawi, MD
Guest Editors
University of Colorado Health Sciences Center
and Univeristy of Colorado Cancer Center
Denver, Colorado
A 56-year-old woman was referred to our institution for a left nephroureterectomy after the diagnoses of a nonfunctioning left kidney and noninvasive papillary urothelial carcinoma of the distal left ureter (Ta grade 1). Following the procedure, surveillance cystoscopy and computed tomography (CT) scan of the abdomen and pelvis demonstrated a large bladder tumor with pan-urothelial extension. A transurethral resection of bladder tumor (TURBT) revealed low-grade papillary urothelial carcinoma without muscle invasion. The patient underwent a cystectomy with a continent ileal diversion to the urethra (neobladder). Approximately 10 months later, a 1.3 × 1.1 cm nodule in the upper lobe of her left lung was seen on CT scan. A video-assisted thoracoscopic surgery (VATS) wedge excision was performed of that lesion. Pathologic evaluation of the lung specimen revealed findings most consistent with low-grade metastatic urothelial carcinoma. Five cycles of a platinum-based regimen were administered after the resection.
Discussion
What did the pathologic examination of the left nephroureterectomy and subsequent cystectomy reveal?
FIGURE 1
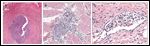
Urothelial Carcinoma of the Urinary Bladder
Dr. Francisco G. La Rosa: This patient shows a very rich surgical pathology history of the urinary tract. Our pathology examination of the left nephrectomy and left ureter specimens revealed a multifocal low-grade urothelial carcinoma present both at the renal pelvis and at the distal portion of the ureter (Figure 1A) and involving the distal ureteral surgical margin. Both of these tumors showed a papillary configuration and were limited to the mucosal surface, thus indicating no evidence of invasion. Three months later, a CT scan showed wide involvement of the urinary bladder by tumor, and tissue from the TURBT revealed low-grade papillary urothelial carcinoma. Following radical cystectomy, examination of the bladder showed a low-grade urothelial carcinoma, but this time with clear evidence of invasion deep into the muscularis propria (American Joint Committee on Cancer [AJCC] stage II: pT2b, N0, MX; Figure 1B) and with multifocal lymphovascular invasion (Figure 1C).
What are the indications for performing a radical cystectomy on a low-grade urothelial carcinoma?
Dr. Shandra Wilson: Indications for cystectomy include stage II–IV bladder cancer, with stage II demonstrating muscularis propria invasion. Radical cystecomy is rare for low-grade cancer. This patient is the only case in 6 years in which we have done a radical cystectomy because of a low-grade urothelial carcinoma. Usually, low-grade urothelial cancers do not tend to become muscle invasive. Alternatively, if tumors recur quickly or cover the entire bladder, then cystectomy is considered. Our patient had both of the latter two conditions.
Dr. Al B. Barqawi: I agree. In this case, the cystectomy specimen revealed a concealed muscle invasive tumor that was missed on the TURBT. A recent review of the literature reported that prior history of transitional cell carcinoma combined with upper tract tumor multifocality were the most frequently reported risk factors for bladder tumors following upper urinary tract urothelial cell carcinomas, which was consistent with this case recurrence.[1]
How often is upper urinary tract cancer associated with urinary bladder cancer?
Dr. Wilson: Urothelial carcinoma is the most prevalent cancer in the upper urinary tract, but only accounts for approximately 5% of all urothelial carcinomas.[2] These upper-tract cancers are thought to be seeding tumors, and various studies have demonstrated the presence of urinary bladder cancer in 15% to 75% of patients within 5 years following an upper-tract diagnosis.[3]
FIGURE 2
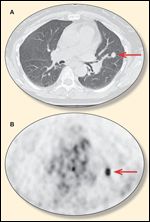
Chest Scans
How does metastatic urothelial carcinoma differ in radiologic appearance in the lung vs a primary lung lesion? Dr. Kimi L. Kondo: There is no specific radiologic characteristic that uniquely distinguishes a urothelial metastasis to the lung from a primary lung cancer. Pulmonary metastases tend to present as multiple lesions, although 5% of all solitary lung nodules are metastatic. Metastases typically have sharp margins and are usually located peripherally and in the lung bases; however, primary lung cancers can also have these characteristics. Nodules with increased 18F-fluorodeoxyglucose (FDG) uptake on positron-emission tomography (PET) are usually thought to be malignant, but inflammatory and infectious processes also can have increased FDG uptake. Biopsy and pathologic analysis are needed to distinguish a urothelial metastasis from a primary lung cancer. In our patient, the CT scan of the chest revealed a nodule with a corresponding hypermetabolic lesion on the FDG-PET scan (Figures 2A, 2B).
The metastatic pulmonary nodule was detected by FDG-PET scan and showed hypermetabolic signs. Is there a role for PET imaging in urothelial carcinoma?
Dr. Thomas W. Flaig: In patients with metastatic disease, I generally initiate staging with a CT scan of the chest, abdomen, and pelvis plus a whole-body bone scan. In this case, an FDG-PET scan demonstrates the malignant deposit in her lung. There are limited data on the usefulness of PET scanning in urothelial cancer. However, the utility of PET scanning was recently examined preoperatively in patients with bladder cancer planning for cystectomy.[4] Occult metastatic disease was detected in 7 of the 42 subjects with negative CT imaging, yielding a sensitivity of 70% and specificity of greater than 90%. This suggests a potential role for PET imaging in urothelial cancer. What has your experience been with resecting metastatic transitional cell carcinoma in the lung?
Dr. J.D. Mitchell: Consultation for metastatic transitional cell carcinoma to the lung is unusual. In my experience, the metastatic nodules attributed to urothelial carcinoma within the lung are typically few in number, peripheral, and amenable to wedge excision.
How do the surgical margins differ when resecting a metastatic lesion in the lung vs a primary lesion?
Dr. Mitchell: When resecting metastatic disease that has spread to the lung, our main goal is complete excision of the lesion with a negative margin. In most cases, this is accomplished with simple wedge excision through a VATS approach. In rare cases, metastatic lesions are not amenable to simple wedge excision, requiring segmentectomy or even lobectomy for complete removal. In modern surgical practice, this too should be done through a VATS approach whenever feasible. Complete removal of all identifiable metastatic lesions is key to the success of the procedure.
In contrast, primary lung cancers are treated preferentially with anatomic lung resection, typically lobectomy, based on data from the Lung Cancer Study Group demonstrating improved local control and a trend toward improved survival with lobectomy compared with sublobar resection.[5] This approach has been challenged in recent years, with excellent results reported in several single-institution studies using sublobar resection for small (< 2 cm) non–small-cell lung cancers.[6,7] A nationwide randomized trial (Cancer and Leukemia Group B [CALGB] 140503) is currently underway, comparing survival and local recurrence rates after lobar vs sublobar resection for lung cancers smaller than 2 cm.
Were there any indications from the nephroureterectomy or bladder pathology that may have suggested future metastasis to the lung?
FIGURE 3
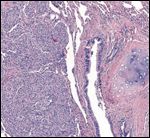
Urothelial Metastasis to the Lung
Dr. La Rosa: Figure 3 demonstrates the urothelial metastasis in the lung. The most evident correlation between the urothelial tumors and the presence of this lung metastasis is the presence of tumor invasion into the muscularis propria and lymphovascular invasion as revealed in the urinary bladder specimen. It is difficult to speculate whether the tumors in the renal pelvis and ureter-which did not show evidence of invasion-had anything to do with this metastasis. Nevertheless, the tumors in all specimens, including the lung metastasis, looked very similar and had the characteristics of low-grade urothelial carcinomas; only a molecular analysis could establish differences between them. Some patients with very limited and noninvasive tumors may present with distant metastasis later in life.[8] However, the presence of muscularis propria invasion and lymphovascular invasion in the bladder specimen make the possibility of distant metastasis most likely.
Would chemotherapy be indicated at this time?
Dr. Flaig: Urothelial carcinoma is a chemotherapy-sensitive cancer. Modern, multidrug chemotherapy regimens have been built around cisplatin, the most active drug in bladder cancer. For many years, our standard approach included the use of the MVAC regimen (methotrexate, vinblastine, doxorubicin, and cisplatin).[9] In the past few years, the activity of gemcitabine (Gemzar) in urothelial carcinoma was recognized. A prospective, randomized comparison of MVAC vs gemcitabine with cisplatin (GC) was performed in patients with advanced urothelial cancer.[10] While the overall 5-year survival was approximately 15% in both arms, many now favor GC over MVAC due to its improved tolerability.
In this case, we have pathologically proven metastatic disease with a suspicious lesion in the pelvis after radical cystectomy for urothelial cancer. The patient’s options at this point would include systemic chemotherapy or observation. The rationale for offering chemotherapy now would be to delay the recurrence of symptomatic metastatic disease. Give the patient’s renal insufficiency, we may have to consider a non–cisplatin-based regimen. Recent measurements of her serum creatinine have ranged from 1.6 to 1.8 mg/dL, and I would estimate her creatinine clearance to be approximately 60 mL/min. An alternative regimen in this setting would include the combination of carboplatin, paclitaxel, and gemcitabine. In the first-line setting, this combination yields an objective response in two-thirds of patients, with approximately half of these being complete responses.[11]
Summary
This 56-year-old patient was initially found to have a papillary, noninvasive, low-grade urothelial carcinoma in the upper urinary tract, which resulted in an obstructive nonfunctioning kidney and a subsequent nephroureterectomy. The carcinoma then manifested in the urinary bladder, and a radical cystectomy was performed. The histology of the urinary bladder revealed low-grade urothelial carcinoma with lymphovascular invasion. Upon PET/CT scan surveillance, a suspicious nodule was found in the upper lobe of the left lung. The patient underwent a left VATS excision that confirmed metastatic low-grade urothelial carcinoma. The patient received five cycles of platinum-based chemotherapy and surveillance imaging with clinic follow-up continued.
Outcome
The patient completed CT scans every 3 months. Upon surveillance, a slow-growing nodule (less than 1cm) was found in each lung, and these were followed conservatively with CT scans. Also, an 11 × 6 cm complex pelvic mass with fluid collection was present on CT scan. The patient underwent an abdominal exploration with removal of the pelvic mass, drainage of fluid, lymph node dissection, and right oopherectomy. The pathology of the mass revealed papillary noninvasive low-grade urothelial carcinoma without lymph node involvement. Three months later, a new suspicious lung nodule was present in the upper lobe of the right lung, while the existing nodules did not show significant changes.
Management Plan
The patient will continue to undergo surveillance CT scans with clinic follow-up every 3 months. Systemic therapy will be reinstituted if significant growth of the pulmonary nodules occurs or the patient has any clinical symptoms. If either of these situations occur, options would include a retrial of carboplatin/docetaxel or a phase I (early human) clinical trial of an investigational agent.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Azémar MD, Comperat E, Richard F, et al: Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: Frequency, risk factors, and surveillance. Urol Oncol Sept 15, 2009 (epub ahead of print).
2. Munoz JJ, Ellison LM: Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J Urol 164:1523-1525, 2000.
3. Flanigan RC: Urothelial tumors of the upper tract, in Wein AJ, Kavoussi LR, Novick AC, et al (eds): Campbell-Walsh Urology, 9th ed, vol 2, p 1640. Philadelphia, Saunders, 2007.
4. Kibel AS, Dehdashti F, Katz MD, et al: Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol 27:4314-4320, 2009.
5. Ginsberg RJ, Rubinstein LV: Randomized trial of lobectomy versus limited resection for T1N0 non-small cell cancer by the Lung Cancer Study Group. Ann Thorac Surg 60:
615-623, 1995.
6. El-Sherif A, Gooding WE, Santos R, et al: Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: A 13-year analysis. Ann Thorac Surg 82:408-415, 2006.
7. Nakamura H, Kawasaki N, Taguchi M, et al: Survival following lobectomy vs limited resection for stage I lung cancer: A meta-analysis. Br J Cancer 92:1033-1037, 2005.
8. Dougherty DW, Gonsorcik VK, Harpster LE, et al: Superficial bladder cancer metastatic to the lungs: Two case reports and review of the literature. Urology 73(1):210.e3-e5, 2009.
9. Sternberg CN, Yagoda A, Scher HI, et al: Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 133:403-407, 1985.
10. von der Maase H, Sengelov L, Roberts JT, et al: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602-4608, 2005.
11. Hussain M, Vaishampayan U, Du W, et al: Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol 19:2527-2533, 2001.