Danazol for the Treatment of Myelodysplastic Syndromes: A Systematic Review
Danazol was reviewed as an effective treatment option for patients with myelodyspplastic syndromes, according to a recently published article by Sangam Shah, MBBS, et al.
ABSTRACT
Purpose
To study the potential utility of danazol for treating patients with myelodysplastic syndromes, with a focus on efficacy and adverse effects (AEs).
Methods
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus were searched for relevant publications from inception June 1, 1950, until June 28, 2022. The studies were screened by title and abstract, followed by full-text screening. The quality of the included studies was assessed via a prespecified set of questionnaires. Data on the efficacy measures and adverse outcomes were extracted and included in a descriptive summary.
Results
Nine studies consisting of 246 participants were included in our review. The overall quality of the included studies was fair. The age of the participants ranged from 61 to 78 years. In all 9 studies, more male patients had been enrolled than female patients. Overall, a proportion of patients in all the studies reported a desired major response to a danazol dose of 400 to 800 mg/day. Few studies did not observe any improvement in the platelet count. Elevated liver enzyme levels, weight gain, headache, dermatitis, and weakness were the most common AEs observed. One study reported a fatal intracerebral hemorrhage in 1 participant.
Conclusions
Danazol has been effective in increasing platelet count and hemoglobin level. Despite a few AEs, danazol is a safe drug for the treatment of patients with myelodysplastic syndromes.
Myelodysplastic syndromes (MDS) are a group of clonal hematological illnesses defined by inefficient hematopoiesis, varying degrees of peripheral cytopenia, and a propensity to develop acute myeloid leukemia (AML).1-3 There is no pharmacologic treatment to cure MDS. Allogeneic stem cell transplant (ASCT) offers a potential cure rate of 30% to 50%,4 but less than 5% of patients are ideal candidates for it. Patients who are not eligible for ASCT have few treatment options.
While a cure for MDS has not yet been found, there have been effective treatments identified to help the population. Azacitidine and decitabine, 2 hypomethylating drugs, have been the first-line therapies advised by the German MDS group for patients with high-risk MDS since 2009.5,6 Comparing the use of azacitidine to best supportive care of erythropoietin has shown that the agent reduces the need for transfusions, lessens the risk of developing AML, and ultimately increases survival.5 In addition, lenalidomide, a microenvironment-modulating drug, has recently been shown to be a successful treatment, especially for patients with low-risk MDS and deletion 5q.6 However, the majority of uninsured patients in low- to middle-income nations are unable to afford these new agents due to their high cost.7 In young patients, high-dose chemotherapy may result in complete remission and a notable improvement.7
For patients with MDS who are older and have poorer performance status and medical comorbidities, supportive care remains the primary treatment.8 Other patient subgroups may respond to growth factors or immunosuppressive medications in varying degrees.9
Danazol is a synthetic androgen with progestational and glucocorticoid properties that inhibits the production of tumor necrosis factor α and IL-1.10,11 Additionally, danazol has proven useful in immunological cytopenias; its androgenic features, which promote healthy hematopoiesis and decrease neoplastic cell clones, are thought to be responsible for its efficacy.11-13 One study noted a 5-year overall survival rate of approximately 60% for patients with aplastic anemia treated with danazol.14
There is, however, conflicting evidence that androgens may be helpful in MDS. Some studies suggest patient benefit, whereas others have demonstrated a lack of efficacy.2,8,9,15,16,18-21 Telomere dysfunction as a pathogenic mechanism in various hematological disorders, particularly in bone marrow failure syndromes, has drawn more attention recently.22,23 Telomeres are noncoding repeating sequences at the end of each DNA chromosome that help maintain chromosomal stability and prevent
chromosomal abnormalities.24
The use of danazol is still a desirable option in a facility with limited access to novel therapeutic alternatives for patients with MDS, such as hypomethylating drugs and lenalidomide, because of its low cost, local availability, and favorable safety profile. Herein, we systematically review the clinical outcomes of patients diagnosed with MDS and treated with danazol as firstline therapy.
Methods
This study’s protocol was created in advance. The systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and its protocol has not been registered in PROSPERO, the international prospective register of systematic reviews.25
Data Sources and Search Strategies
MEDLINE In-Process & Other Non- Indexed Citations, MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus were all thoroughly searched electronically for relevant pieces of literature published from inception June 1, 1950, until June 28, 2022. The 2 study authors independently planned and carried out the search method. The appendix contains a full description of the search strategy (Supplemental File 1).
Study Selection
Each article’s eligibility was evaluated by 2 separate authors (SS and RY) based on predetermined standards after carefully reading all the titles and abstracts. We retrieved the pertinent references’ entire texts and uploaded them for full-text evaluation in accordance with the qualifying requirements. Conflicts were settled by consensus and with the help of a third reviewer (KG).
Eligibility Criteria
In this systematic review, we incorporated randomized controlled trials, original papers (cohort, prospective study), and studies that examined the use of danazol to treat patients with MDS. Studies had to report at least 1 of the following outcomes:
- Major response
- Minor response
- Platelet count
According to the study procedures, major response, minor response, increase in platelet count, and hematologic parameters were all defined. Case reports, editorials, reviews, and articles written in a language other than English were excluded.
Data Extraction
Two reviewers (SS and RY) utilized Microsoft Excel, 2013 version, to create standardized, pilot-tested forms and independently extracted data (2013). Discussions between the 2 reviewers helped settle disagreements. Study characteristics, participant descriptions, intervention information, and important outcomes were retrieved from each study. A number of relevant patient outcomes were extracted, and we gathered information on the results at the longest follow-up period mentioned in the study.
Quality Assessment
The quality assessment of the included studies was performed by the authors (AB and SS) in accordance with the following items: (1) clarity of the study objectives; (2) study period stated clearly; (3) criteria for patient selection; (4) study conducted in multiple centers; (5) danazol treatment method and dosage mentioned; (6) baseline equivalence groups clearly considered; (7) definition of the primary outcome (major response, minor response, or platelet count defined prior to the study; (8) adequate follow-up period; (9) adverse effects (AEs) stated; and (10) the limitations of each study were considered. We did not use quality assessment as an exclusion criterion. Individual study questions were answered with yes or no, with 1 point awarded for yes and 0 points for no. Total score was calculated for each study. The quality of the included studies was judged to be fair (total score, 8-10), average (5-7), and low (0-4). Consensus with the reviewer (RY) was used to resolve any disputes.
Synthesis of Results
The narrative synopsis with summary tables for attributes covered all identified studies. Additionally, descriptive statistics were used to summarize the data. For dichotomous variables, we used frequencies and percentages, whereas we used mean or median for continuous variables.
Outcome of Interest
Efficacy Measures
The following were used to examine patients’ functional outcomes:
- Major response was defined as:
- a more than 2-g/dL increase in hemoglobin level for patients with a pretreatment hemoglobin level of less than 11 g/dL;
- transfusion independence for patients who were red blood cell (RBC) transfusion dependent;
- an absolute increase of 30 Å~ 109/L or more for patients with a pretreatment platelet count of less than 100 Å~ 109/L;
- stabilization of platelet count and platelet transfusion independence for patients who were platelet transfusion dependent; or
- a 100% increase or an absolute neutrophil count (ANC) increase of more than 0.5 Å~ 109/L for patients with a pretreatment ANC of less than 1.5 Å~ 109/L
2. Minor response was defined as:
- an increase of 1 to 2 g/dL in hemoglobin level for patients with a pretreatment hemoglobin level of less than 11 g/dL;
- a 50% decrease in transfusion requirements for patients who were RBC transfusion dependent;
- a 50% or more increase in platelet count with a net increase greater than 10 Å~ 109/L but less than 30 Å~ 109/L; or
- an ANC increase of at least 100% but an ANC increase of less than 0.5 Å~ 109/L.26 Response was required to last at least 2 months.
3. Increase in platelet count:
Response was defined as a platelet count increase of at least 30 Å~ 109/L.
Results
Literature Search and Study Selection
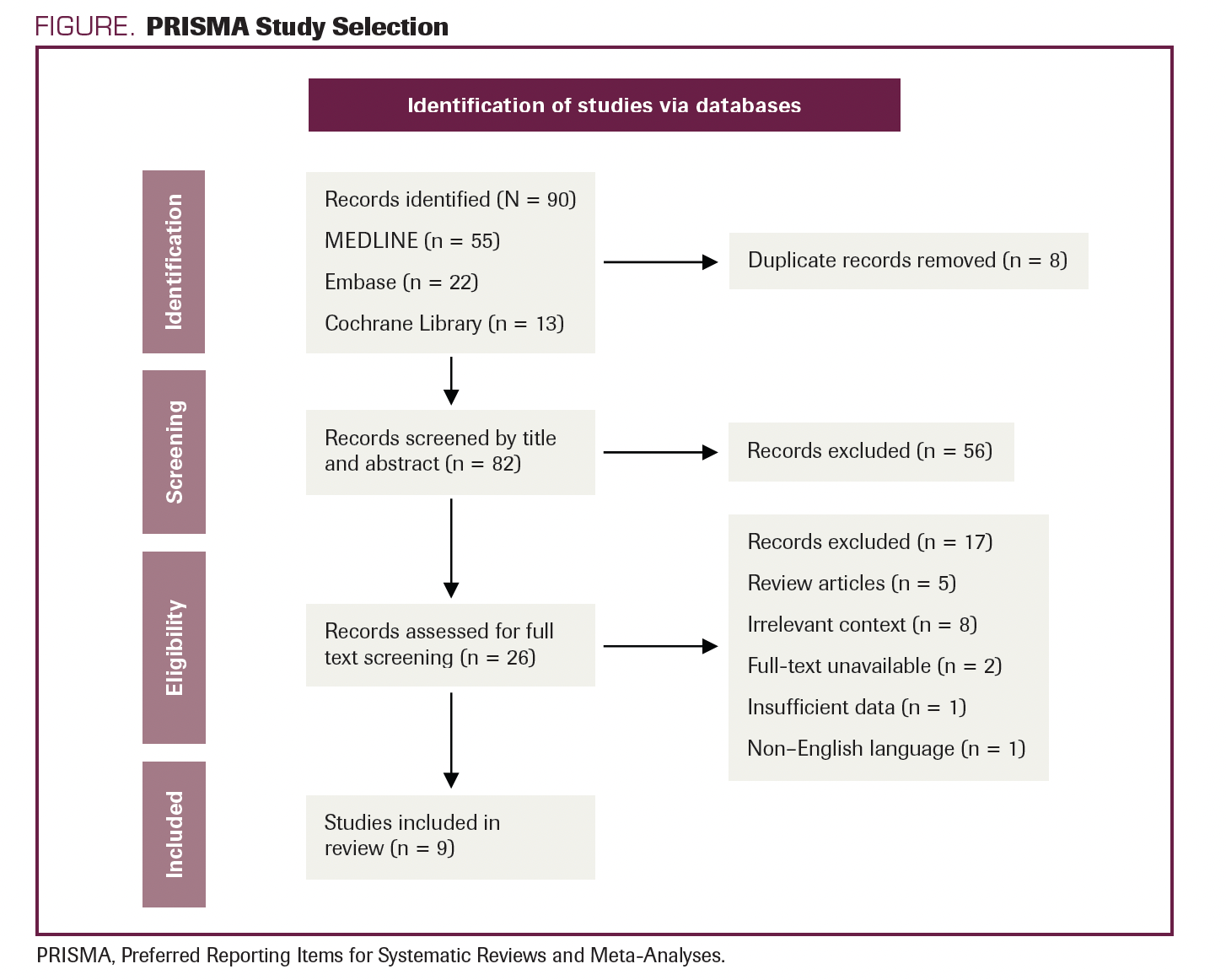
The literature search resulted in the retrieval of 90 studies. After the complete screening process of titles, abstracts, and full texts, 81 studies did not meet the eligibility criteria. Nine articles with various study designs that met the criteria were included in the review. A description of study selection is shown in the PRISMA flow diagram in the Figure.
FIGURE. PRISMA Study Selection
Quality Assessment Among the Included Studies
The result of the quality assessment of the included studies is displayed in Table 1.2,11,17-20,24-26 We included and critically analyzed a total of 9 studies, among which 6 scored 10 of 10, 2 scored 9 of 10, and 1 scored 8 of 10. Based on our judgments, all studies scored between 8 and 10 and were therefore deemed fair quality. The average score was 9.55, making the overall quality of the included studies fair.
TABLE 1. Quality Assessment of the Included Studies2,11,17-20,24-26
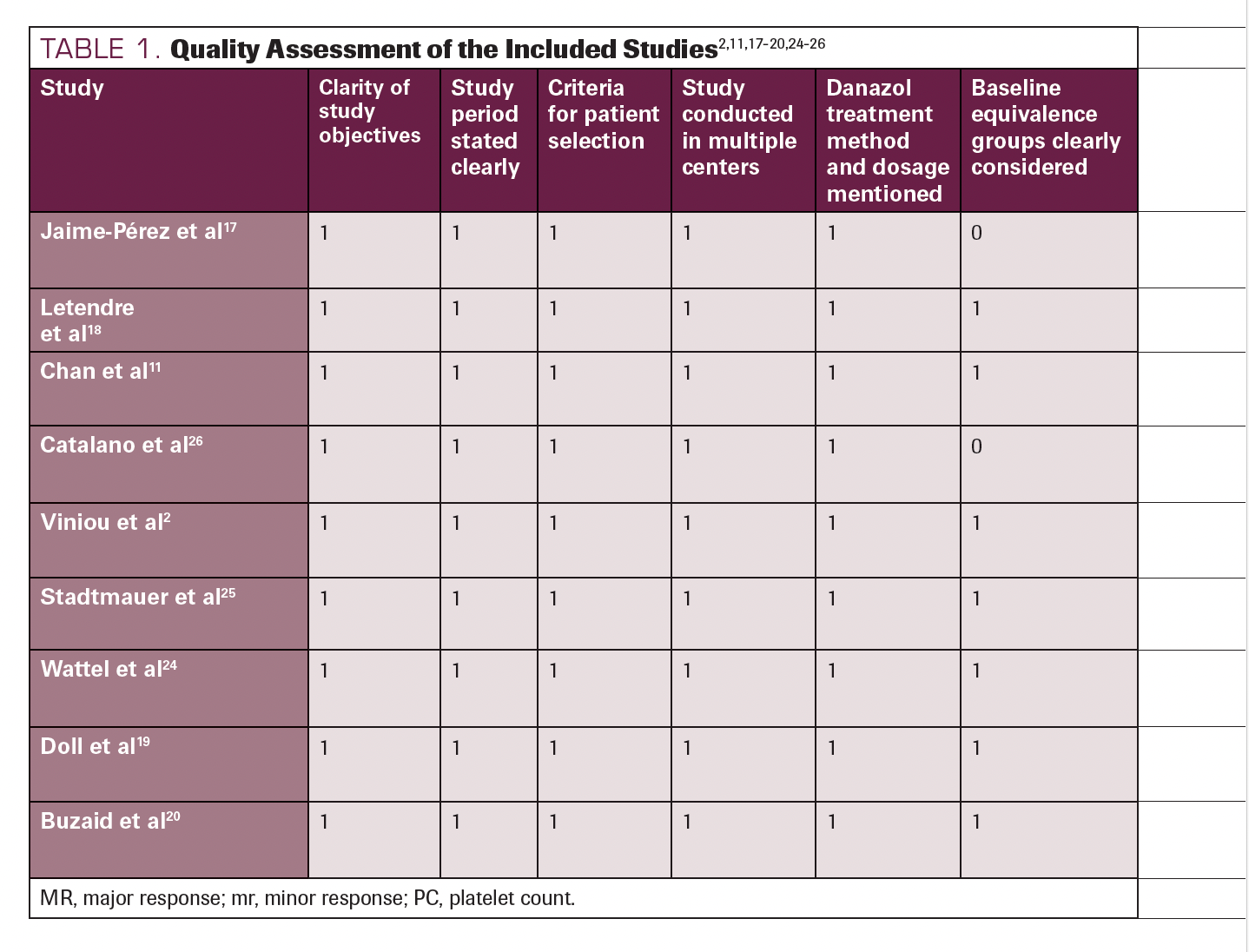
TABLE 1. Continued
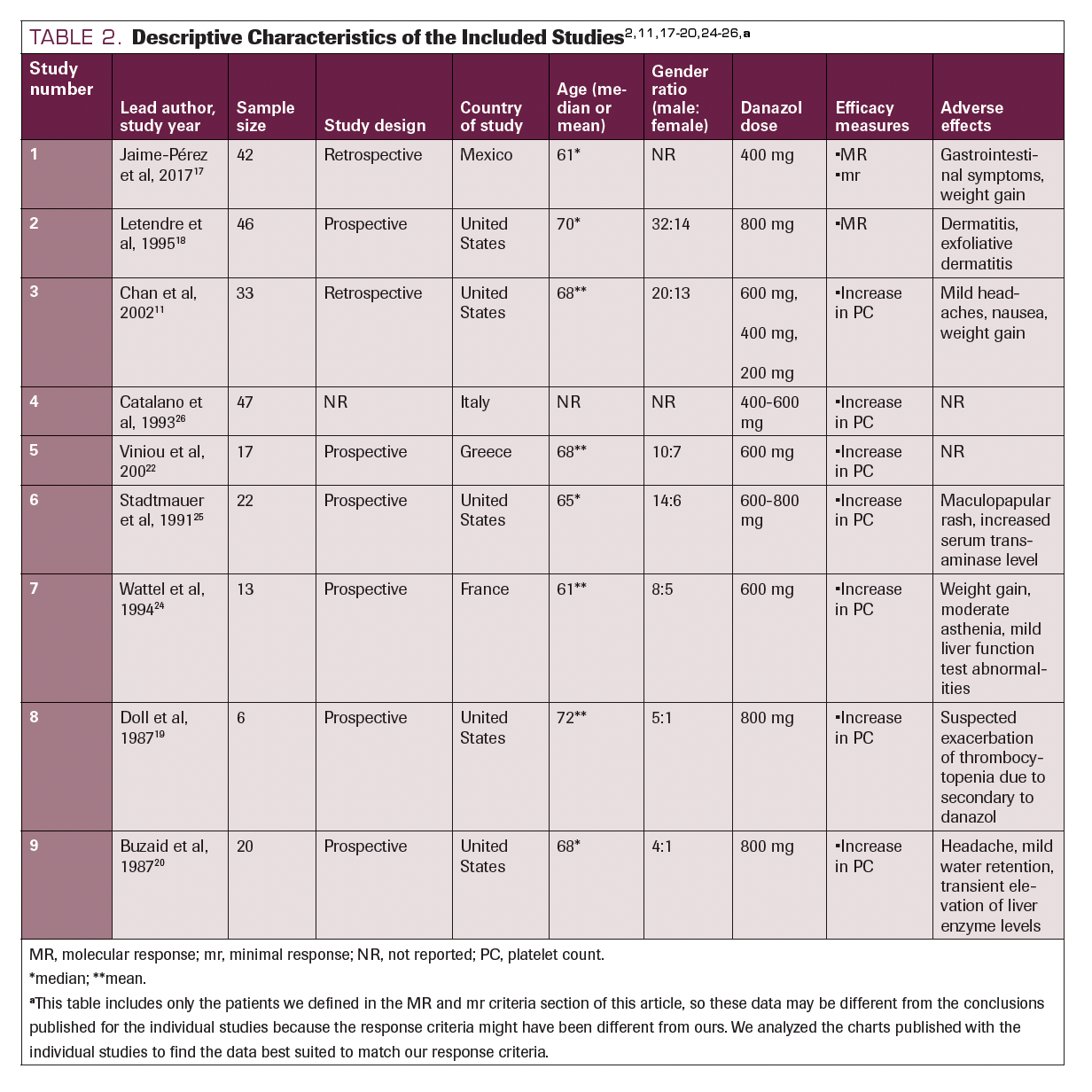
Descriptive Characteristics of the Included Studies
Nine studies consisting of 246 participants were included in our review. Six were prospective observation studies, and the remaining 4 studies were retrospective. Six studies were performed in North America (5 of which in the United States), and 3 studies were conducted in Europe. The age of the participants ranged from 61 to 72 years, and all 9 studies enrolled more male than female patients. All the studies reported the dose of danazol administered and at least 1 of the efficacy measures (major response, minor response, or increased platelet count). Seven studies reported the AEs of danazol observed in the patients. The descriptive characteristics of the 9 included studies are detailed in Table 2.2,11,17-20,24-26
TABLE 2. Descriptive Characteristics of the Included Studies2,11,17-20,24-26,a
Efficacy Measures
Overall, the participants (with MDS subtypes refractory anemia [RA], RA with ringed sideroblasts [RARS], RA with excess of blasts [RAEB], RAEB in transformation [RAEB-T], or chronic myelomonocytic leukemia [CMML]) in all 9 studies experienced the desired response, and their hemoglobin level and platelet count increased. In studies from which we could derive data, 41.37% of patients in the RA group, 40% in the CMML group, 35.4% in the RAEB group, 10% in the RAEB-T group, and 4% in RARS group responded with either a major or a minor response. All studies except Buzaid et al followed the participants for 3 months.20
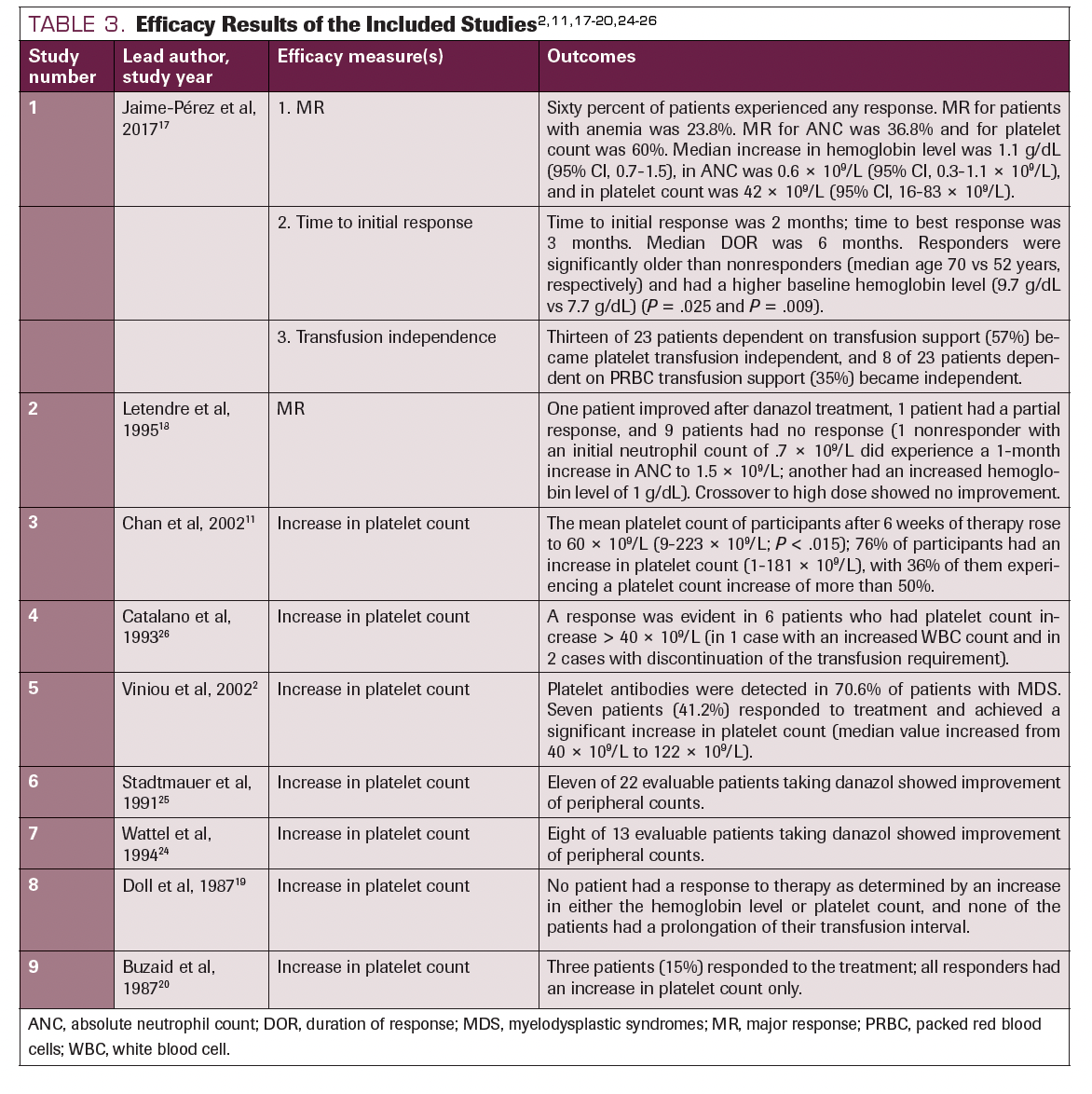
In Catalano et al, 6 of 47 patients who received 400 to 600 mg of daily danazol showed a major response in platelet count and 1 of 47 and 2 of 47 patients showed a major response in ANC and hemoglobin level, respectively. 26 Similar results were demonstrated by Chan et al—10 of 33 patients administered 200 to 600 mg of daily danazol showed a major response in platelet count.11 Major responses were satisfactorily obtained in all studies that mentioned it. In the study done by Jaime-Pérez et al, more than half of the participants experienced an increased platelet count.14 In the study performed by Buzaid et al, all the participants who did not improve had RAEB.20 Stadtmauer et al and Letendre et al both reported an elevated hemoglobin level as the minor response achieved.18,25 However, the findings were contrary in Doll et al.19 None of the participants showed an increased platelet count at a dose of 800 mg/day of danazol. Table 32,11,17-20,24-26 details the outcomes of each study.
TABLE 3. Efficacy Results of the Included Studies2,11,17-20,24-26
Adverse Effects
Elevated liver enzyme levels, weight gain, headache, dermatitis, and weakness were the most common AEs observed among the participants treated with danazol. In Buzaid et al, 2 patients discontinued the drug before 4 weeks, 1 due to a fatal intracerebral hemorrhage and 1 due to intractable headache, which improved after discontinuation of the drug.20 Doll et al19 reported thrombocytopenia in the participants, but the etiology was not determined and attributed to exacerbation secondary to danazol administration. The AEs experienced by participants in the included studies are shown in Table 2.
Discussion
Different drugs have been developed to treat patients with MDS, but stem cell transplantation is the only potential cure available; however, there is no cure for the vast majority of patients with MDS.27 As a result, treatment for patients with MDS aims to delay the onset of AML in high-risk patients and reduce cytopeniarelated problems in low-risk patients.28
MDS has been treated with corticosteroids, 13-cis-retinoic acid, and androgens with varying degrees of success.10,29,30 Hypomethylating drugs have recently been found to improve hematological outcomes in 25% to 50% of patients with high-risk MDS, achieve a full response in 10% to 20% of patients, and enhance survival compared with best supportive therapy.31 Despite these hopeful outcomes, 40% to 50% of patients will not benefit from the hypomethylating drugs.31 Due to its effectiveness in treating immunological cytopenia, danazol has drawn particular attention. Additionally, it has recently been demonstrated that the agent can lengthen telomeres, resulting in a hematological response in patients with telomere illness.32 However, earlier research on danazol as a treatment for MDS had conflicting outcomes.32
Since the 1960s, androgens such as danazol have been used to treat MDS and AML.19 Because clonal myeloid disorders like MDS, AML, and aplastic anemia share anomalies of the TERC/ TERT partners of telomerase activity, one hypothesis for the positive effect of androgens such as danazol on telomerase may explain why norethandrolone has recently been shown to be effective in treating AML.33
Danazol’s mode of action in patients with MDS is still unknown. Many of our patients’ responses took longer than expected, which raises the possibility that the mechanism of action may be immune mediated rather than involving direct stimulation of megakaryocytes. People with idiopathic thrombocytopenic purpura (ITP) and autoimmune hemolytic anemia respond well to danazol therapy.34 Danazol also has been shown to lower the antiplatelet IgG level in patients with ITP who are responding.35,36 A decrease in the quantity of platelet-associated IgG may not be the only mechanism through which the response to danazol is mediated. 37 Numerous immunological problems, such as the development ofautoantibodies and autoimmune diseases, are linked to MDS,38 and immunosuppressive therapy is effective in certain patients with MDS.39 The delay in platelet response in certain patients after 12 weeks of danazol therapy suggests an immune-mediated impact that may necessitate a prolonged course of treatment, even though danazol’s mechanism of action in MDS and immune-mediated illnesses is unknown.
Limitations
Our review had several limitations. First, neither a worse prognosis nor clinical signs were used to choose individuals for the majority of the research. Danazol was used concurrently with other medications, which also affected the results. The efficacy end points that were utilized to measure effectiveness might be unreliable for a variety of MDS. Additionally, the functional scale used to evaluate efficacy varied between studies. The included research also had tiny sample numbers, and the study’s time frame was likewise brief. Finally, most of the trials were single center, open label, and nonrandomized.
Conclusions
Our systematic review included a total of 246 patients; 57 (23.17%) of these patients showed a major response in platelet count, 18 (7.32%) had a major response in ANC, and 15 (6.10%) experienced a major response in hemoglobin level. Two patients showed a minor response in ANC, and 1 patient had a minor response in hemoglobin level. Danazol has been effective in increasing platelet count and hemoglobin level. Despite a few AEs, danazol is a safe treatment option for patients with MDS.
DISCLOSURE: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
AUTHOR AFFILIATIONS:
1Institute of Medicine, Tribhuvan University, Kathmandu, Nepal
2Kist Medical College, Kathmandu, Nepal
3Saint Mary Mercy, Livonia, Michigan
4Morehouse School of Medicine, Atlanta, Georgia
5Department of Internal Medicine, University of
Nebraska Medical Center
Corresponding author
Krishna Gundabolu, MBBS, MS
Department of Internal Medicine
University of Nebraska Medical Center
Email: krishna.gundabolu@unmc.edu
References
- Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: contemporary review and how we treat. Am J Hematol. 2016;91(1):76-89. doi:10.1002/ajh.24253
- Viniou N, Plata E, Terpos E, et al. Danazol therapy for thrombocytopenia in patients with myelodysplastic syndromes. Acta Haematol. 2002;107(4):234-236. doi:10.1159/000058321
- Harnan S, Ren S, Gomersall T, et al. Association between transfusion status and overall survival in patients with myelodysplastic syndromes: a systematic literature review and meta-analysis. Acta Haematol. 2016;136(1):23-42. doi:10.1159/000445163
- Colunga-Pedraza PR, Colunga-Pedraza JE, Garza- Ledezma MA, et al. Danazol as first-line therapy for myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2018;18(2):e109-e113. doi:10.1016/j. clml.2017.11.007
- Sekeres MA, Taylor J. Diagnosis and treatment of myelodysplastic syndromes: a review. JAMA. 2022;328(9):872-880. doi:10.1001/jama.2022.14578
- Götze K, Platzbecker U, Giagounidis A, et al. Azacitidine for treatment of patients with myelodysplastic syndromes (MDS): practical recommendations of the German MDS Study Group. Ann Hematol. 2010;89(9):841-850. doi:10.1007/s00277-010-1015-0
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higherrisk myelodysplastic syndromes: a randomised, openlabel, phase III study. Lancet Oncol. 2009;10(3):223-232. doi:10.1016/S1470-2045(09)70003-8
- Shaw RP, Griffin CC, Escobar M-L. The Impact of Health Insurance in Low- and Middle-Income Countries. Brookings Institution Press; 2010.
- List A, Dewald G, Bennett J, et al; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456-1465. doi:10.1056/NEJMoa061292
- Hellström-Lindberg E. Treatment of adult myelodysplastic syndromes. Int J Hematol. 1999;70(3):141-154.
- Chan G, DiVenuti G, Miller K. Danazol for the treatment of thrombocytopenia in patients with myelodysplastic syndrome. Am J Hematol. 2002;71(3):166-171. doi:10.1002/ajh.10209
- Sadek I, Zayed E, Hayne O, Fernandez L. Prolonged complete remission of myelodysplastic syndrome treated with danazol, retinoic acid and low-dose prednisone. Am J Hematol. 2000;64(4):306-310. doi:10.1002/1096-8652(200008)64:4<306::aid-ajh12>3.0.co;2-v
- Cines DB, Cassileth PA, Kiss JE. Danazol therapy in myelodysplasia. Ann Intern Med. 1985;103(1):58-60. doi:10.7326/0003-4819-103-1-58
- Audia S, Godeau B, Bonnotte B. Is there still a place for “old therapies” in the management of immune thrombocytopenia? Rev Med Interne. 2016;37(1):43-49. doi:10.1016/j.revmed.2015.08.007
- Liu W, Gu X, Fu R, et al. The effect of danazol in primary immune thrombocytopenia: an analysis of a large cohort from a single center in China. Clin Appl Thromb Hemost. 2016;22(8):727-733. doi:10.1177/1076029615622002
- Avilés A. A review of 76 patients with myelodysplastic syndromes treated with danazol. Cancer. 1995;75(1):133-134. doi:10.1002/1097-0142(19950101)75:1<133::aidcncr2820750124>3.0.co;2-i
- Jaime-Pérez JC, Colunga-Pedraza PR, Gómez- Ramírez CD, et al. Danazol as first-line therapy for aplastic anemia. Ann Hematol. 2011;90(5):523-527. doi:10.1007/s00277-011-1163-x
- Letendre L, Levitt R, Pierre RV, et al. Myelodysplastic syndrome treatment with danazol and cis-retinoic acid. Am J Hematol. 1995;48(4):233-236. doi:10.1002/ ajh.2830480405
- Doll DC, Ringenberg QS, Yarbro JW. Danazol therapy in acquired idiopathic sideroblastic anemia. Acta Haematol. 1987;77(3):170-171. doi:10.1159/000205984
- Buzaid AC, Garewal HS, Lippman SM, Durie BG, Katakkar SB, Greenberg BR. Danazol in the treatment of myelodysplastic syndromes. Eur J Haematol. 1987;39(4):346-348. doi:10.1111/j.1600-0609.1987. tb00780.x
- Najean Y, Pecking A. Refractory anaemia with excess of myeloblasts in the bone marrow: a clinical trial of androgens in 90 patients. Br J Haematol. 1977;37(1):25-33.
- Najean Y, Pecking A. Refractory anemia with excess of blast cells: prognostic factors and effect of treatment with androgens or cytosine arabinoside. results of a prospective trial in 58 patients. Cooperative Group for the Study of Aplastic and Refractory Anemias. Cancer. 1979;44(6):1976-1982. doi:10.1002/1097 0142(197912)44:6<1976::aidcncr2820440603>3.0.co;2-#
- Hupperets P, De Witte T, Haanen C. A retrospective study of the effect of anabolic steroids on the dyshaematopoietic syndrome (preleukaemic syndrome). Neth J Med. 1983;26(7):181-187.
- Wattel E, Cambier N, Caulier MT, Sautière D, Bauters F, Fenaux P. Androgen therapy in myelodysplastic syndromes with thrombocytopenia: a report on 20 cases. Br J Haematol. 1994;87(1):205-208. doi:10.1111/j.1365-2141.1994.tb04895.x
- Stadtmauer EA, Cassileth PA, Edelstein M, et al. Danazol treatment of myelodysplastic syndromes. Br J Haematol. 1991;77(4):502-508. doi:10.1111/j.1365-2141.1991.tb08617.x
- Catalano L, Selleri C, Montuori N, et al. Danazol for myelodysplastic syndromes. Br J Haematol. 1993;85(1):230-231. doi:10.1111/j.1365-2141.1993. tb08681.x
- Young NS. Bone marrow failure and the new telomere diseases: practice and research. Hematology. 2012;17(suppl 1):S18-S21. doi:10.1179/10245331 2X13336169155132
- Colla S, Ong DS, Ogoti Y, et al. Telomere dysfunction drives aberrant hematopoietic differentiation and myelodysplastic syndrome. Cancer Cell. 2015;27(5):644-657. doi:10.1016/j.ccell.2015.04.007
- Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27(5):791-798. doi:10.1200/JCO.2008.17.1033
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. doi:10.1016/j. jclinepi.2009.06.005
- Cheson BD, Bennett JM, Kantarjian H, et al; World Health Organization (WHO) international working group. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671-3674.
- Gordon MS. Advances in supportive care of myelodysplastic syndromes. Semin Hematol. 1999;36(6) (suppl 6):21-24.
- Ahn YS. Efficacy of danazol in hematologic disorders. Acta Haematol. 1990;84(3):122-129. doi:10.1159/000205048
- Huang HE, Lin KM, Lin JC, et al. Danazol in refractory autoimmune hemolytic anemia or immune thrombocytopenia: a case series report and literature review. Pharmaceuticals (Basel). 2022;15(11):1377. Published 2022 Nov 9. doi:10.3390/ph15111377
- Motoji T, Teramura M, Takahashi M, et al. Successful treatment of refractory anemia with high-dose methylprednisolone. Am J Hematol. 1990;33(1):8-12. doi:10.1002/ajh.2830330103
- Marini B, Bassan R, Barbui T. Therapeutic efficacy of danazol in myelodysplastic syndromes. Eur J Cancer Clin Oncol. 1988;24(9):1481-1489. doi:10.1016/0277-5379(88)90339-2
- Zeidan AM, Kharfan-Dabaja MA, Komrokji RS. Beyond hypomethylating agents failure in patients with myelodysplastic syndromes. Curr Opin Hematol. 2014;21(2):123-130. doi:10.1097/ MOH.0000000000000016
- Townsley DM, Dumitriu B, Liu D, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374(20):1922-1931. doi:10.1056/ NEJMoa1515319
- Pigneux A, Béné MC, Guardiola P, et al. Addition of androgens improves survival in elderly patients with acute myeloid leukemia: a GOELAMS study. J Clin Oncol. 2017;35(4):387-393. doi:10.1200/ JCO.2016.67.6213

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.