Emerging Treatments and Evolving Paradigms in HER2-Low Breast Cancer
During a continuing medical education activity hosted by PER, Paolo Tarantino, MD, discussed HER2-low breast cancer and emerging treatment options to fight the disease.
HER2-low metastatic breast cancer (mBC) represents a recently established subset of HER2-negative (HER2–) BC, defined by a HER2 immunohistochemical (IHC) score of 1+ or 2+ and in situ hybridization (ISH) negative phenotype.1 Recent clinical trial data have shown clinical and survival benefit with novel, HER2-targeting antibody-drug conjugates (ADCs). Specifically, fam-trastuzumab deruxtecan-nxki (T-DXd) is the first FDA-approved targeted therapy for HER2-low BC based on the phase 3 DESTINY-Breast04 trial.2
It is estimated that approximately 45% to 55% of patients with BC are classified as HER2-low; however, HER2 scoring criteria varies.1 Interestingly, HER2-low status is more common in patients with hormone receptor–positive (HR+) BC than in those with triple-negative breast cancer (TNBC).
In this article, Paolo Tarantino, MD, advanced research fellow at the Breast Oncology Center at Dana-Farber Cancer Institute and Harvard Medical School in Boston, Massachusetts, discusses biologic insights, the current treatment landscape, and relevant data updates for HER2-low BC.
Q: Which HER2-targeted therapies have shown efficacy in HER2-low BC? How do novel anti-HER2 therapies challenge the HER2 binary paradigm?
TARANTINO: This is a very interesting question. It began with the idea that we may be able to expand the reach of HER2-targeted treatments beyond the 15% to 20% of patients who have HER2+ disease to the larger population with HER2-low BC (at least IHC 1+).3 The largest trial that tested this hypothesis was NSABP B-47, a phase 3 study examining whether adding trastuzumab to adjuvant chemotherapy improves outcomes in the curative setting for patients with early-stage HER2-low BC.4 This study was negative in terms of disease-free and overall survival (OS), demonstrating that blocking HER2 with a naked antibody does not benefit patients with HER2-low BC.5 Years after the presentation of these results, linking agents to antibodies such as chemotherapy with ADCs, was found to provide relevant antitumor activity in both HER2+ and HER2-low BC.6
This was not seen with trastuzumab emtansine (T-DM1), likely because it has few chemotherapy molecules per antibody (drug to antibody ratio).7 The drug to antibody ratio of T-DM1 is 3.5, whereas novel ADCs have up to 8 molecules of chemotherapy attached and utilize cleavable linkers and novel mechanisms like topoisomerase inhibitors.8 Several novel ADCs have shown activity in HER2-low disease. The only approved ADC is trastuzumab deruxtecan (T-DXd), but others (like trastuzumab duocarmazine) have also demonstrated activity.9 Most notably, a compound from China called SHR-A1811 had response rates above 50% in metastatic HER2+ and HER2-low BC.10 We expect these and other conjugates may be active in HER2-low disease, but, to date, ADCs have shown the most promise while naked antibodies and tyrosine kinase inhibitors have had insufficient activity.
Q: How has the approval of T-DXd changed the way you treat HER2-low BC? How has this approval affected patient outcomes?
TARANTINO: The approval of T-DXd for patients with HER2-low BC occurred very rapidly, just 2 months after the data were presented at the 2022 American Society of Clinical Oncology Annual Meeting.2 This approval has greatly impacted how we treat these patients since few drugs provide an OS advantage in HER2– mBC, which progresses after endocrine therapy and chemotherapy. With a 50% response rate and significant benefits in progression-free survival (PFS) and OS, we can be confident that this drug will help patients in the clinic, which is why it has been widely adopted as a preferred second-line agent for HER2-low metastatic disease.11 I have seen prolonged responses, and we hope to present real-world data soon. The impact of T-DXd is huge, not only due to its activity, but also because of the ability to treat a large population. HER2-low cancers account for over half of all breast cancers.1
Q: Interstitial lung disease (ILD)/pneumonitis is an adverse event of interest with T-DXd. How do you manage ILD in patients undergoing treatment with T-DXd therapy?
TARANTINO: It is extremely important to be aware of the ILD risk with T-DXd. Approximately 10% to 15% of patients receiving the drug are expected to develop some degree of ILD or pneumonitis, although, in most cases, it is only grade 1 or 2.12 Grade 1 refers to radiographic findings only, while grade 2 includes mild symptoms. In most T-DXd trials, there were also some fatal cases, usually 1% or less of patients, which reminds us of the severity of this adverse effect. Importantly, risk does not appear cumulative, but is highest within the first year of treatment. The median onset of ILD is about 4 to 5 months after starting T-DXd.
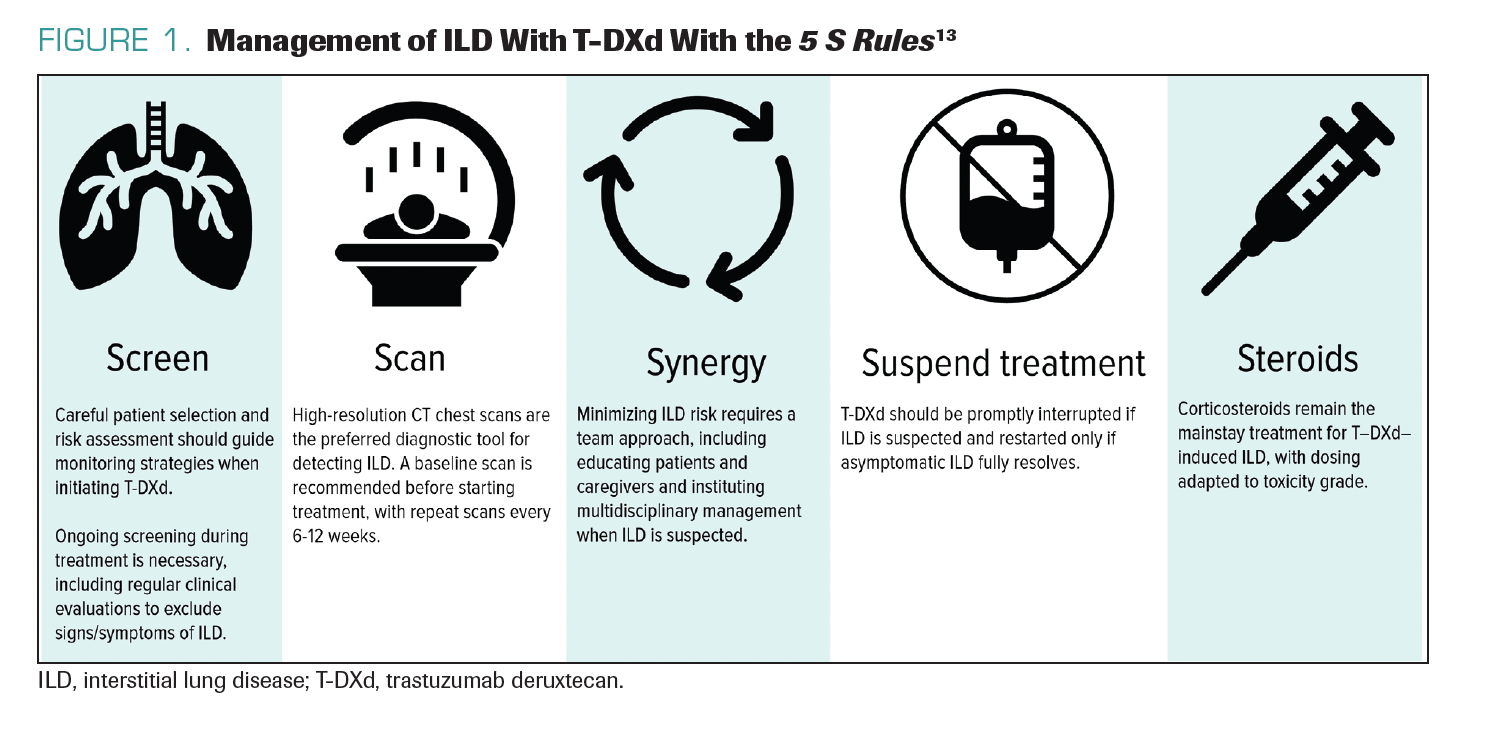
A helpful framework is the 5 S Rules tomonitor and manage ILD: (1) screening to understand patient risk factors (eg, comorbidities, frailties, or vulnerabilities); (2) scanning with serial chest CTs at 6 to 12 weeks for lower-risk patients and preferably at 6 to 9 weeks for high-risk patients; (3) synergy in discussing cases with radiologists and pulmonologists to establish multidisciplinary care; (4) suspension of treatment with any suspicion of ILD or permanent discontinuation of treatment with T-DXd if the ILD is symptomatic; and (5) steroids for treatment (Figure 1).13 Steroids are the mainstay of treatment, and patients require access to oral or intravenous (IV) steroids to manage ILD. With this approach, fatal cases decreased from 2.7% in the DESTINY-Breast01 trial to 0% in the DESTINY-Breast03 trial.14,15 Management is getting better, but there are still some cases of ILD in certain clinical trials, as well as in
clinical practice.
FIGURE 1. Management of ILD With T-DXd With the 5 S Rules13
Q: Can you please comment on the updated survival data from the DESTINY-Breast04 trial presented at the European Society for Medical Oncology Congress 2023?
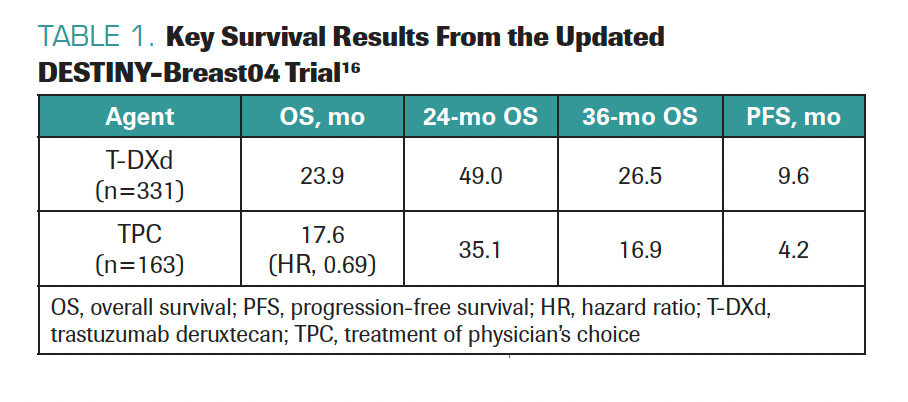
TARANTINO: It was nice to see the update of the DESTINY-Breast04 trial. The results confirmed what we already knew: T-DXd works much better than chemotherapy, in terms of PFS and OS, with medians of survival quite consistent with those observed at the prior presentation of the data. T-DXd, compared with chemotherapy, roughly doubled PFS and achieved an OS benefit of about 6 months (Table 1).16
TABLE 1. Key Survival Results From the Updated DESTINY-Breast04 Trial16
Q: Are there any other treatment approaches being evaluated in patients with HER2-low BC?
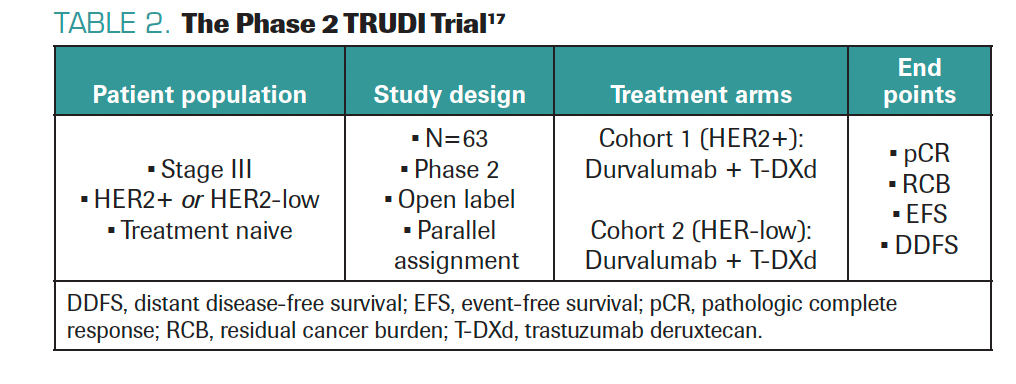
TARANTINO: After seeing the impact of T-DXd in the metastatic setting, we want to explore the potential in the curative setting. Can T-DXd cure more patients with early-stage or locally advanced HER2+ or HER2-low BC? Can T-DXd prevent metastatic recurrence? This led to testing T-DXd with durvalumab as neoadjuvant treatment for inflammatory breast cancer with HER2 expression in the phase 2 TRUDI trial (Table 2).17 I helped design this trial with Filipa Lynce, MD, at Dana-Farber; the trial is open and accruing patients at Dana-Farber and The University of Texas MD Anderson Cancer Center. We hope to see a high pathologic complete response rate with this combination in HER2+ and HER2-low disease, where there is major unmet need. We hope to have data within the next few years.
TABLE 2. The Phase 2 TRUDI Trial17
Additional trials in the curative setting are ongoing, like DESTINY-Breast05 and DESTINY-Breast11 in HER2+ settings, and the TRIO-US B-12 TALENT trial in HER2-low settings, which reported early activity at San Antonio Breast Cancer Symposium 2022 last year.18-20 Many more trials are ongoing, so there are opportunities to leverage this potent drug.
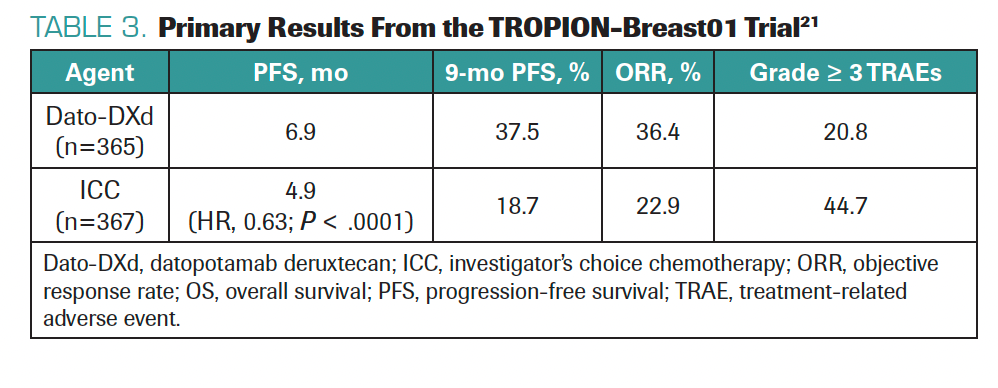
We are also considering drugs like datopotamab deruxtecan (Dato-DXd), which is a similar ADC that uses the deruxtecan payload but targets TROP2 instead of HER2.21 Primary data presented at ESMO 2023 showed positive survival outcomes compared with chemotherapy in the TROPION-Breast01 trial in patients with HR+/HER2– BC (Table 3).21 Dato-DXd could be FDA approved next year; this would raise sequencing challenges but provide more treatment options. Since ADCs have distinct adverse effects, it is good to adapt treatment strategy, activity, and toxicities to patient preferences and profiles. In the future, we may have multiple ADCs to select from based on a multiplicity of patient- and disease-related factors.
TABLE 3. Primary Results From the TROPION-Breast01 Trial21
Q: How do you test for HER2 in your practice?
TARANTINO: We test for HER2 in the classical way according to the ASCO/College of American Pathologists (CAP) guidelines, which is the authority for HER2 testing and interpretation on all samples with IHC and ISH/fluorescence in situ hybridization (FISH).22 These allow us to determine if a tumor is HER2-positive or negative, with negative meaning no amplification or overexpression, not complete absence. IHC can also identify HER2-low tumors with 1+ or 2+ staining without amplification.
Some institutions do not perform IHC and perform only ISH/FISH, which misses HER2-low status. The 2023 ASCO/CAP guideline update specified adding a footnote that some ADCs can be used in HER2-low BC.22 In general, following ASCO/CAP guidelines and performing IHC and FISH testing on tissue is the most comprehensive. We may have novel assays in the future, including blood-based tests to determine HER2 status from plasma, but we’re not there yet.
Q: What genetic differences exist between HER2-low and HER2-zero tumors?
TARANTINO: When the HER2-low subgroup was defined and established in practice, a key question was whether it is a distinct molecular subtype of BC or just a clinical entity without molecular basis. Several groups studied this by comparing the genomic profiles of HER2-low and HER2-zero tumors. We presented one of the largest datasets on this at SABCS 2022, comparing gene mutations, amplifications, and copy number variations in more than 1000 patients with mBC.23 We found no significant differences after multiple testing corrections.
The only difference was the average ERBB2 allele copy number, which was higher in HER2-low tumors and which may potentially have therapeutic repercussions.23 Single-copy ERBB2 deletions were more common in HER2-zero than in HER2-low BC. This was the only major difference; HER2-low and HER2-zero are not considered distinct molecular entities, but they exist as part of a spectrum. We are now trying to dissect this spectrum with quantitative mRNA and proteomic assays.
Q: Which biopsy should be used to define a tumor as HER2-low?
TARANTINO: This is a major clinical dilemma in treating these patients, because we tend to trust the most recent biopsy as reflecting the current biology of the tumor. But, with HER2-low, this is tricky due to the high discordance rate between primary and recurrent tumors, and between subsequent biopsies over time. About 30% of tumors change from HER2-zero to HER2-low or vice versa at each time point.24 Given this variability and heterogeneity, results of a Dutch autopsy study found different liver lesions in the same patient range from HER2-low to HER2-zero to HER2–ultra-low, depending on the biopsy site.25, 26
The biopsy result dictates treatment and it is not always consistent. Given this and the OS benefit of T-DXd, we have become pragmatic on how we treat patients. The ESMO expert consensus on HER2-low breast cancer had over 90% agreement on using any biopsy in the patient’s history for T-DXd consideration.27 Even if only 1 of many is HER2-low, even if it is the primary or an old biopsy, it may predict T-DXd benefit over chemotherapy.
A DESTINY-Breast04 subgroup analysis examined whether primary tumor status predicted the benefit of T-DXd in the metastatic setting and whether an older biopsy predicted benefit as well as a recent one.28 The answer was yes in both cases. The benefit of T-DXd over chemotherapy was consistent regardless of the tissue used for enrollment. Currently, we favor using any biopsy to consider T-DXd eligibility. We will present data on HER2-low evolution and T-DXd activity, so these assumptions may evolve as we learn more about their relationship over time.
Q: The J101, DESTINY-Breast01, and BEGONIA trials excluded patients with HER2-zero tumors. How does the phase 2 DAISY trial differ and what is the key takeaway from that trial?
TARANTINO: It was bold to design large trials like DESTINY-Breast04 that treated historically HER2– disease with an ADC. Designing for HER2 1+ and 2+/ISH-negative disease led to an effective treatment for these patients. The same occurred in the phase 1 J101 trial, BEGONIA, and most trials utilizing T-DXd in patients with HER2– and HER2-low expression.29,30
The phase 2 DAISY trial included cohorts for HER2+, HER2-low, and HER2-zero disease treated with T-DXd.31 This showed encouraging activity not just in HER2+ and HER2-low disease, but in HER2-zero disease as well, demonstrated by a 30% objective response rate in patients with HER2-zero and disease progression on chemotherapy. It also showed PFS was dependent upon HER2 expression; the longest PFS was in HER2+ patients, intermediate PFS was seen in HER2-low, and the shortest PFS was in patients with HER2-zero disease.
This makes sense, because chemotherapy can detach from the antibody and circulate in the body like traditional chemotherapy, meaning that T-DXd likely has some activity irrespective of HER2 expression. This may explain activity in metastatic HER2-zero disease. If HER2 is expressed or overexpressed, there is additional, more durable activity of T-DXd. Overall, the DAISY trial expanded the horizon beyond what we consider HER2-expressing BC, and patients with HER2-zero disease may potentially benefit from T-DXd.
Q: How does the trial design of the phase 3 DESTINY-Breast06 trial differ from that of the DESTINY-Breast04 trial? What are the implications of these data and how can we use these data moving forward?
TARANTINO: DESTINY-Breast04 was a second-line trial for HR+/HER2– BC progressing on endocrine treatment and at least 1 line of chemotherapy.16 This trial demonstrated superior outcomes with T-DXd compared with chemotherapy. The phase 3 DESTINY-Breast06 trial was initiated to evaluate T-DXd vs taxanes or capecitabine among patients with HR+ metastatic disease progressing on endocrine therapy without prior chemotherapy.32
There are 2 additional key differences from DESTINY-Breast04. First, about 10% of patients in DESTINY-Breast04 had triple-negative disease, whereas DESTINY-Breast06 investigators limited eligibility to HR+ disease.32,33 Second, DESTINY-Breast04 included only HER2-low patients, while DESTINY-Breast06 also included those with HER2–ultra-low disease (IHC 0 with < 10% HER2 expression). If the data are positive, DESTINY-Breast06 could expand the use of T-DXd to the first-line setting following endocrine therapy failure and to patients with HER2–ultra-low expression. The results of this important trial may substantially broaden the use of T-DXd.
Q: In your opinion, what does the future hold with respect to the evolution of HER2-low therapies? What is on the horizon that is shaping the treatment paradigm and the future of care in this space?
TARANTINO: An important part of the future will be determined by biomarkers, because IHC is not ideal for assessing HER2-low disease. IHC identifies HER2+ amplified/overexpressed cancer well, but, in the HER2-low realm, T-DXd had equal activity in IHC 1+/2+ and ISH-negative disease in DESTINY-Breast04.33 IHC does not predict T-DXd activity, and we need better assays. Immunofluorescence, mass spectrometry, mRNA analysis, liquid biopsy, and more are being tested. Finding a biomarker to predict T-DXd activity in HER2-low disease will help to optimize treatment.
Other ADCs beyond T-DXd are being tested in this space. Disitamab vedotin is an ADC with a microtubule inhibitor payload.34 Another ADC with a topoisomerase I inhibitor payload, SHR-A1811, has shown promising activity in preliminary trials.10 In general, many ADCs may fill this space, leading to sequencing challenges to determine optimal treatment strategies. A major question is whether ADCs like T-DXd could replace anthracyclines and taxanes for early-stage treatment. Ongoing and planned trials will help answer if T-DXd and other ADCs can provide better cures for early BC, which is a huge innovation opportunity, because we need better curative treatments for these patients.
Q: In your opinion, what is one of the biggest unmet needs in breast oncology?
TARANTINO: One of the biggest unmet needs in breast oncology is effective treatment for brain metastases. It has been encouraging to see novel ADCs achieve high intracranial response rates and efficacy in this population. Recent data at ESMO 2023 showed a pooled analysis of DESTINY-Breast01, DESTINY-Breast02, and DESTINY-Breast03
trials that demonstrated response rates of over 40% in patients with HER2+ disease and untreated or active brain metastases.35 PFS reached 1 year with stable brain metastases and 18.5 months with active brain metastases.
In general, promising data indicate that we are moving in the right direction to better treat patients with brain metastases. We still lack data on T-DXd for HER2-low brain metastases. The DEBBRAH trial had very few of these patients so more data are needed.36
We know that HER2+ BC has a high brain metastasis incidence; 30% to 50% of patients develop brain metastasis.37 Brain metastasis is less frequent in HER2– disease, but because most breast cancers are HER2–, in absolute terms, most brain metastasis patients we see clinically have HER2– disease.38
We have yet to see much benefit for these patients with traditional chemotherapy, but ADCs are moving the needle. At Dana-Farber Cancer Institute, we will open a phase 2 trial of Dato-DXd for patients with brain metastases (DATO-BASE trial), with either HR+ or triple-negative disease and even leptomeningeal disease, which has a very poor prognosis.39 Even in this setting, we can see responses with T-DXd and other ADCs, so it is important to study ADCs in this population. We hope to see activity for these difficult-to-treat patients.
Taken together, several emerging agents are shaping the evolving treatment landscape in defining and treating HER2-low mBC.
References
- Zhang H, Peng Y. Current biological, pathological and clinical landscape of HER2-low breast cancer. Cancers (Basel). 2022;15(1)doi:10.3390/cancers15010126
- FDA approves fam-trastuzumab deruxtecan-nxki for HER2-low breast cancer. News release. FDA. August 5, 2022. Accessed October 19, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer#:~:text=low%20breast%20cancer-,FDA%20approves%20fam%2Dtrastuzumab%20deruxtecan%2Dnxki,for%20HER2%2Dlow%20breast%20cancer&text=On%20August%205%2C%202022%2C%20the,%2C%20Daiichi%20Sankyo%2C%20Inc
- O'Shaughnessy J, Gradishar W, O'Regan R, Gadi V. Risk of recurrence in patients with HER2+ early-stage breast cancer: Literature analysis of patient and disease characteristics. Clin Breast Cancer. 2023;23(4):350-362. doi:10.1016/j.clbc.2023.03.007
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-52. doi:10.1200/JCO.2014.55.5730
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673-84. doi:10.1056/NEJMoa052122
- Fan P, Xu K. Antibody-drug conjugates in breast cancer: Marching from HER2-overexpression into HER2-low. Biochim Biophys Acta Rev Cancer. 2023;1878(1):188849. doi:10.1016/j.bbcan.2022.188849
- Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105-117. doi:10.1016/S0140-6736(22)02420-5
- Abelman RO, Medford A, Spring L, Bardia A. Antibody-drug conjugates in breast cancer: Spotlight on HER2. Cancer J. 2022;28(6):423-428. doi:10.1097/PPO.0000000000000634
- Banerji U, van Herpen CML, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124-1135. doi:10.1016/S1470-2045(19)30328-6
- Yao H, Yan M, Tong Z, et al. Safety, tolerability, pharmacokinetics, and antitumor activity of SHR-A1811 in HER2-expressing/mutated advanced solid tumors: A global phase 1, multi-center, first-in-human study. Cancer Res. 2023;83(suppl 8):CT175. doi:10.1158/1538-7445.AM2023-CT175
- NCCN. Clinical Practice Guidelines in Oncology. Breast cancer, version 4.2023. Accessed October 31, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Fam-trastuzumab deruxtecan-nxki. Prescribing information. Daiichi Sankyo Co., Ltd.; 2022. Accessed November 7, 2023. https://daiichisankyo.us/prescribing-information-portlet/getPIContent?productName=Enhertu&inline=true
- Tarantino P, Tolaney SM. Detecting and managing T-DXd-related interstitial lung disease: The five "S" rules. JCO Oncol Pract. 2023;19(8):526-527. doi:10.1200/OP.23.00097
- Modi S, Saura C, Yamashita T, et al. Updated results from DESTINY-Breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd ) in HER2 positive metastatic breast cancer. Cancer Res. 2021;81(suppl 4):PD3-06. doi:10.1158/1538-7445.SABCS20-PD3-06
- Cortes J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143-1154. doi:10.1056/NEJMoa2115022
- Modi S, Jacot W, Iwata H, et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Updated survival results of the randomized, phase III DESTINY-Breast04 study. Ann Oncol. 2023;34(suppl 2):334-335. doi:10.1016/j.annonc.2023.09.553
- TRUDI: T-DXd+Durva in HER2+/Low IBC. ClinicalTrials.gov. Updated October 10, 2023. Accessed November 2, 2023. https://clinicaltrials.gov/study/NCT05795101
- Charles E Geyer J, Untch M, Prat A, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) vs trastuzumab emtansine (T-DM1) in high-risk patients with HER2-positive, residual invasive early breast cancer after neoadjuvant therapy: A randomized, phase 3 trial (DESTINY-Breast05). Cancer Res. 2021;81(suppl 4):OT-03-01. doi:10.1158/1538-7445.SABCS20-OT-03-01
- Harbeck N, Boileau J-F, Modi S, et al. A phase 3, open-label trial of neoadjuvant trastuzumab deruxtecan (T-DXd) monotherapy or T-DXd followed by THP compared with ddAC-THP in patients with high-risk HER2-positive early-stage breast cancer (DESTINY-Breast11). Cancer Res. 2022;82(suppl 4):OT1-12-04. doi:10.1158/1538-7445.SABCS21-OT1-12-04
- Bardia A, Hurvitz S, Press MF, et al. GS2-03 TRIO-US B-12 TALENT: Neoadjuvant trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early stage breast cancer Cancer Res. 2023;83(suppl 5):GS2-03. doi:10.1158/1538-7445.SABCS22-GS2-03
- Bardia A, Jhaveri K, Im S, et al. Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer (BC): Primary results from the randomised phase III TROPION-Breast01 Ann Oncol. 2023;34(suppl 2):S1254-S1335. doi:10.1016/annonc/annonc1358
- Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline update. J Clin Oncol. 2023;41(22):3867-3872. doi:10.1200/JCO.22.02864
- Schettini F, Chic N, Braso-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi:10.1038/s41523-020-00208-2
- Miglietta F, Griguolo G, Bottosso M, et al. HER2-low breast cancer: Evolution from primary breast cancer to relapse. Ann Oncol. 2021;32(suppl 2):S23. doi:10.1016/j.annonc.2021.03.018
- Hoefnagel LD, van der Groep P, van de Vijver MJ, et al. Discordance in ERalpha, PR and HER2 receptor status across different distant breast cancer metastases within the same patient. Ann Oncol. 2013;24(12):3017-23. doi:10.1093/annonc/mdt390
- Geukens T, Schepper MD, Richard F, et al. HER2-16 Inter-lesion heterogeneity of HER2-status in metastatic breast cancer: possible implications for treatment with anti-HER2 antibody-drug conjugates. Cancer Res. 2022;83(suppl 5):HER2-16. doi:10.1158/1538-7445.SABCS22-HER2-16
- Tarantino P, Viale G, Press MF, et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol. 2023;34(8):645-659. doi:10.1016/j.annonc.2023.05.008
- Prat A, Modi S, Tsurutani J, et al. HER2-18 Determination of HER2-low status in tumors of patients with unresectable and/or metastatic breast cancer in DESTINY-Breast04. Cancer Res. 2023;83(suppl 5):HER2-18. doi:10.1158/1538-7445.SABCS22-HER2-18
- Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512-1522. doi:10.1016/S1470-2045(17)30604-6
- Schmid P, Wysocki P, Ma CX, et al. Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): updated results from BEGONIA, a phase 1b/2 study. Abstract presented at: SABCS 2022; December 6-10, 2022; San Antonio, TX. Accessed December 16, 2022.
- Diéras V, Deluche E, Lusque A, et al. Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY) Cancer Res. 82(suppl 4):PD8-02. doi:10.1158/1538-7445.SABCS21-PD8-02
- Bardia A, Barrios C, Dent R, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice of chemotherapy in patients with hormone receptor-positive (HR+), HER2 low metastatic breast cancer whose disease has progressed on endocrine therapy in the metastatic setting: A randomized, global phase 3 trial (DESTINY-Breast06). Cancer Res. 2021;81(suppl 4):OT-03-09. doi:10.1158/1538-7445.SABCS20-OT-03-09
- Harbeck N, Modi S, Jacot W, et al. Trastuzumab deruxtecan vs treatment of physician’s choice in patients with HER2-low unresectable and/or metastatic breast cancer: Subgroup analyses from DESTINY-Breast04. Cancer Res. 2023;83(suppl 5):P1-11-01. doi:10.1158/1538-7445.SABCS22-P1-11-01
- Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022;29(1):1335-1344. doi:10.1080/10717544.2022.2069883
- Hurvitz SA, Modi S, Li W. A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINY-Breast (DB) -01, -02, and -03. Ann Oncol. 2023;34(suppl 2):S335-S336. doi:10.1016/j.annonc.2023.09.554
- Pérez-García JM, Batista MV, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol. 2023;25(1):157-166. doi:10.1093/neuonc/noac144
- Garcia-Alvarez A, Papakonstantinou A, Oliveira M. Brain metastases in HER2-positive breast cancer: Current and novel treatment strategies. Cancers (Basel). 2021;13(12)doi:10.3390/cancers13122927
- Patel SH, Saito YD, Li Z, et al. A solitary brain metastasis as the only site of recurrence of HR positive, HER2 negative breast cancer: a case report and review of the literature. J Med Case Reports. 2021;15(4)doi:10.1186/s13256-020-02615-2
- Dana Farber Cancer Institute. DATO-BASE: A phase 2 trial of DATOpotamab-deruxtecan for breast cancer Brain metAstaSEs. Updated 2023. Accessed November 7, 2023. https://www.dana-farber.org/clinical-trials/23-533
Oncology Journal Online Article
Emerging Treatments and Evolving Paradigms in HER2-Low Breast Cancers
Release Date: December 1, 2023
Expiration Date: December 1, 2024
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Activity Overview
This continuing medical education (CME) activity provides expert insight regarding HER2-low breast cancer (BC). This program discusses biologic insights, the current treatment landscape, and relevant data updates for HER2-low BC.
Acknowledgment of Support
This activity is funded by PER®.
Instructions for Participation/How to Receive Credit
Complete the activity (including pre- and post-activity assessments).
Answer the evaluation questions.
Request credit using the drop-down menu.
You may immediately download your certificate.
Start Online Activity
Learning Objectives
Upon successful completion of this activity, you should be better prepared to:
Identify patients with HER2-low breast cancer by utilizing guideline-based recommendations to differentiate between HER2-negative and HER2-low breast cancer
Discuss the rationale for and clinical relevance of recent clinical trials utilizing HER2-targeting strategies for the management of patients with HER2-low breast cancer
Determine strategies to integrate evolving evidence into treatment planning for patients with HER2-low breast cancer
Faculty
Paolo Tarantino, MD
Advanced Research Fellow
Breast Oncology Center
Dana-Farber Cancer Institute
Harvard Medical School
Boston, MA
This activity was written by PER® editorial staff under faculty guidance and review. The Q&A portion of the activity was transcribed from a recorded interview with the faculty and edited by faculty and PER® editorial staff for clarity.
Faculty, Staff, and Planners’ Disclosures
In accordance with ACCME Guidelines, PER® has identified and resolved all COI for faculty, staff, and planners prior to the start of this activity by using a multistep process.
Disclosures (Dr Tarantino): Consultant: AstraZeneca, Daiichi Sankyo, Genentech, Gilead, Lilly, Roche.
The staff of PER® have no relevant financial relationships with commercial interests to disclose.
Off-Label Disclosure/Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a healthcare professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER® or any of the companies that provided commercial support for this activity.
CME Provider Contact Information
Physicians’ Education Resource®, LLC
2 Clarke Drive, Suite 110
Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com
