Defining the Role of Hepatic Arterial Infusion Chemotherapy in Metastatic Colorectal Cancer
The use of hepatic arterial infusion (HAI) chemotherapy in patientswith liver-only colorectal metastases is based on the pharmacologicprinciple that the regional administration of some drugs can lead tohigher drug concentrations at the site of the metastases and avoid systemictoxicity. Early randomized trials resulted in high response ratesbut did not lead to a survival advantage with HAI. More recent trialshave utilized improved surgical techniques and strict guidelines regardingdose reduction or cessation of HAI chemotherapy, resulting in asignificant reduction in toxicity. In patients with unresectable liver metastases,two recent European trials using HAI fluorouracil (5-FU)again failed to demonstrate an improvement in survival, but both wereplagued by a high complication rate with an associated high proportionof patients failing to receive the assigned treatment. In contrast,the preliminary results of a recent Cancer and Leukemia Group B trialdid demonstrate a survival advantage with HAI floxuridine when comparedto systemically administered 5-FU. Trials investigating the useof HAI chemotherapy in the adjuvant setting have yielded mixed results.Moreover, in light of improved response rates and overall survivalwith newer more active chemotherapeutic and novel agents, theabsolute role of HAI chemotherapy remains undefined.
ABSTRACT: The use of hepatic arterial infusion (HAI) chemotherapy in patientswith liver-only colorectal metastases is based on the pharmacologicprinciple that the regional administration of some drugs can lead tohigher drug concentrations at the site of the metastases and avoid systemictoxicity. Early randomized trials resulted in high response ratesbut did not lead to a survival advantage with HAI. More recent trialshave utilized improved surgical techniques and strict guidelines regardingdose reduction or cessation of HAI chemotherapy, resulting in asignificant reduction in toxicity. In patients with unresectable liver metastases,two recent European trials using HAI fluorouracil (5-FU)again failed to demonstrate an improvement in survival, but both wereplagued by a high complication rate with an associated high proportionof patients failing to receive the assigned treatment. In contrast,the preliminary results of a recent Cancer and Leukemia Group B trialdid demonstrate a survival advantage with HAI floxuridine when comparedto systemically administered 5-FU. Trials investigating the useof HAI chemotherapy in the adjuvant setting have yielded mixed results.Moreover, in light of improved response rates and overall survivalwith newer more active chemotherapeutic and novel agents, theabsolute role of HAI chemotherapy remains undefined.In the United States, approximately140,000 people will be diagnosedwith colorectal cancer in2004, with one-third ultimately dyingof metastases from that disease. Mostmetastases occur in the liver, andaround 80% of patients with hepaticmetastases will die of progressive liverinvolvement.In most instances, colorectal cancermetastasizes to multiple sites.However, metastases may be limitedto the liver in a significant subset ofpatients. This is evidenced by longtermdisease-free survival in manypatients who undergo resection of isolatedhepatic metastases. Unfortunately,although one-third of patients withhepatic metastases have disease confinedto the liver, resection is possiblein only 10% of these patients due toextensive infiltrative disease. Of thissmall group, roughly 30% will realizelong-term survival with resection.[1]Although normal hepatocytes receivetheir blood supply from the portalcirculation, liver metastases receive80% of their blood supply via thehepatic artery.[2] The recognition ofthis feature of hepatic tumors led toregional delivery of chemotherapy byway of the hepatic artery, preferentiallydelivering drug to the tumorwhile sparing normal hepatocytes.Extensive investigation of hepaticarterial infusion (HAI) chemotherapybegan in the 1970s using percutaneouslyplaced catheters. With the introductionof a totally implantablepump in the 1980s,[3] further investigationsuggested a marked improvementin response rates but failed toshow a significant survival benefit.Techniques employed in pump placementand administration of chemotherapywere then further refined inan effort to improve safety and optimizeresponse and survival.Not every chemotherapeutic agenthas the pharmacologic properties oractivity in colorectal cancer to justifyits HAI use. Floxuridine (FUDR) hasbecome the agent of choice becauseof a relatively high hepatic-to-systemicratio (400) and high rate of hepaticextraction (94% to 99%). Fluorouracil(5-FU) has also been commonly used,although it has a lower hepatic-tosystemicratio (10 to 100) and rate ofhepatic extraction (20% to 55%).[4,5]Additionally, the relative insolubilityof that 5-FU precludes administrationusing an implantable pump (sinceit must be diluted in a relatively largevolume), requiring the use of a portor percutaneously placed catheterinstead.
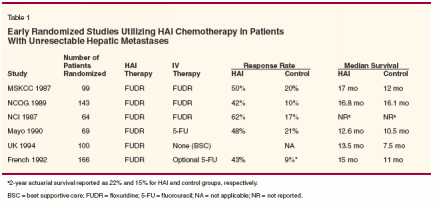
Randomized studies have employedHAI chemotherapy as primarytherapy in patients with unresectablehepatic metastases as well asin the adjuvant setting after resectionof liver metastases. This article willsummarize these studies and addresscurrent issues related to the practicaladministration and future role of HAIchemotherapy in an era of improvedefficacy and survival with newer chemotherapeuticand novel agents.Primary Treatment forUnresectable Liver MetastasesIn the early 1980s, with only 5-FUor its analogs available, four majorprospective trials involving 375 patientscompared systemic fluoropyrimidinechemotherapy with HAIFUDR. Although the response rate was consistently superior in the HAIarm of all four trials, none was able todemonstrate a statistically significantsurvival advantage.[6-9] Two Europeantrials in the late 1980s randomizedpatients with unresectable livermetastases to receive HAI FUDR orbest supportive care. Both trials demonstratedan improved survival advantageamong patients treated withHAI chemotherapy. However, mostpatients in the control arms did notreceive systemic chemotherapy, whichwould be considered suboptimal treatmentby today's standards (see Table1).[10,11]Two meta-analyses have been performedto address the possibility thatthe lack of a survival benefit in thesestudies reflected sample size limitationsrather than treatment failure.[12,13] Both confirmed thesuperior response rate with HAI chemotherapycompared to systemic chemotherapy.However, while there wasa trend toward improved survival inthe HAI arms, this survival benefitonly reached statistical significancewhen including those patients whoreceived no treatment in the controlarms.Data from these trials and the subsequentmeta-analyses consistentlydemonstrate a significant improvementin response with HAI chemotherapy.However, this improvementin response rate also consistently failedto translate into a significant survivaladvantage. Although several factorsmay be responsible for this apparentdisconnect, surgical issues associatedwith pump placement and hepatobiliarytoxicity associated with chemotherapyadministration were found tobe significant contributors to HAItreatment's failure to achieve a survivalbenefit.[14,15]Refinement of Surgical Techniqueand Chemotherapy Delivery
The goal of pump placement istwofold: (1) to achieve complete hepaticperfusion of chemotherapy, and(2) to prevent misperfusion of chemotherapyto the stomach or duodenumwith its resultant inflammationand ulcer formation. Initial studiessuggested a relatively high incidenceof misperfusion and lack of completehepatic perfusion. This problem wasaddressed with an attempt to standardizepump placement technique ina manner that would minimize theseproblems.[16]Several safeguards are now routinelyimplemented to ensure completehepatic arterial patency andperfusion and to exclude misperfusion.Preoperative angiography is performedto evaluate anomalous hepaticperfusion and confirm portal vein patency.Angiography is now followedby exploratory laporatomy to excludeextrahepatic disease with electivecholecystectomy to prevent postoperativechemical cholecystitis. Intraoperatively,the pump port is injectedwith fluorescein and the liver, stomach,and duodenum are examined usinga Wood's lamp to ensureappropriate perfusion. Finally, priorto using the pump, radionuclide angiography(with technetium macroaggregatedalbumin) is employedthrough the pump catheter, again toensure adequate hepatic perfusion andexclude misperfusion.In addition to standardization ofsurgical techniques, different chemotherapeuticregimens have been employedto minimize drug-inducedhepatic toxicity. The dose of FUDRused in most of the above studies (0.3mg/kg/d) resulted in a high proportionof patients experiencing permanentbiliary damage. Further phase IIstudies explored different dosingschedules as well as using dexamethasoneand/or leucovorin to minimizetoxicity or increase efficacy.Combining HAI chemotherapy withsystemic chemotherapy has also beenaddressed with similar goals.HAI Therapy:Maximizing FUDR?
One phase II trial conducted atUniversity of California, San Francisco(UCSF), used a much lower infusionalFUDR dose alternating withHAI bolus 5-FU, seeking to take advantageof the differing toxicities andpharmacokinetics of these drugs. Innone of the 64 patients treated wasadministration of this regimen limitedby hepatobiliary toxicity. In thisgroup, where 30 had not responded toprior systemic chemotherapy, the HAIregimen described resulted in a 50%objective response rate with a mediansurvival of 22.4 months. However,progressive liver tumor was the initialsite of failure and cause of death inthe majority of patients.[17]A series of studies performed atMemorial Sloan-Kettering CancerCenter (MSKCC) sought to better definethe optimal administration of HAIFUDR. One randomized phase IIstudy attempted to minimize biliaryinflammation with its associated hepatobiliarytoxicity by administeringdexamethasone with FUDR. Thisstudy failed to show a decrease intoxicity or increase in drug delivery,but surprisingly suggested an improvementin response rate and mediansurvival in the dexamethasone armcompared to FUDR alone.[18] A similarattempt to increase efficacy andreduce toxicity included varying dosesof leucovorin in combination withvarying doses of FUDR. Biliary sclerosisremained high, but again, longtermsurvival was better thanpredicted.[19]Ultimately, MSKCC created a hybridregimen combining FUDR, dexamethasone,and leucovorin in aneffort to both reduce toxicity and maximizeresponse. In phase II trials, theresponse rate using this regimen inpreviously untreated patients was 78%with a median survival of 24.8 months.Using strict criteria for dose reductionor discontinuation of treatment,the incidence of biliary sclerosis wasalso reduced to 3%.[20]Employing these techniques, surgicalcomplications and chemotherapeutictoxicity have improvedmarkedly. Prior to these refinements,the incidence of biliary sclerosisranged from 10% to 40% with an incidenceof ulcer formation or gastritisfrequently over 20%. With the safeguardsdescribed above, the incidenceof biliary sclerosis is now less than5% with a similar reduction in ulcerformation or gastritis. In addition tothe above-mentioned surgical safeguards,European investigators haveemployed 5-FU rather than FUDRwith similarly stringent guidelines regardingdose reduction and discontinuationof treatment. This hasresulted in similar improvement intreatment-related toxicity, althoughthe use of external catheters and portsresults in a greater incidence of catheterocclusion and hepatic artery thrombosis.Recent Studies
Two large European studies andone United States study have recentlybeen reported using HAI chemotherapyas the primary treatment modalityfor patients with unresectablecolorectal hepatic metastases (seeTable 2).
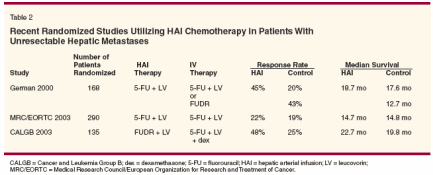
The German Cooperative Groupon Liver Metastases published resultsfrom a multicenter randomized studywhere 168 patients were assigned toone of three arms: (1) HAI 5-FU plusleucovorin, (2) IV 5-FU plus leucovorin,or (3) HAI FUDR. In terms ofresponse and survival, there were nostatistically significant differencesamong the three treatment arms. Intrahepaticresponse was similar in thetwo HAI treatment arms (45% with5-FU, 43% with FUDR) and, as expectedfrom previous trials, lower inthe IV 5-FU arm (20%). On the otherhand, median survival was similar inthe HAI and IV 5-FU arms (18.7 and17.6 months, respectively), with mediansurvival in the HAI FUDR armlower at 12.7 months (not statisticallysignificant).[21]The Medical Research Council andEuropean Organization for Researchand Treatment of Cancer (MRC/EORTC) colorectal cancer studygroups randomized 290 patients toreceive either HAI 5-FU plus leucovorinor systemic 5-FU plus leucovorin.In contrast to previous studies,response rates were similar betweenthe HAI and IV arms (22% vs 19%).Median survival also did not differbetween the two groups (14.7 vs 14.8months for the HAI and IV groups,respectively).[22]Both European trials used 5-FU asthe chemotherapeutic agent deliveredvia the hepatic artery. The reasonscited for choosing 5-FU were twofold:(1) to reduce hepatobiliary toxicityassociated with FUDR; and (2)to take advantage of 5-FU's lowerhepatic extraction rate with its associatedsystemic spillover, ostensiblytreating systemic micrometastatic disease.Because of relative insolubilityand the associated necessary largevolume, administering HAI 5-FU requiresusing percutaneously placedcatheters or ports rather than implantablepumps. In multiple reviews, portshave been associated with a significantlyhigher complication rate comparedto implantable pumps.[23,24]Additionally, without the use of continuousheparin infusion as is usedwith implantable pumps, the use ofports or catheters has resulted in ahigher incidence of hepatic arterythrombosis.In the German study, 32% of patientsassigned to the HAI arms failedto receive their assigned therapy. Similarly,the MRC/EORTC trial authorspoint out that 37% of patients assignedto the HAI arm did not receive theirdesignated treatment in this trial. Additionally,only two cycles were givenon average in the HAI arm of thistrial because of the associated toxicity.This high rate of patients failing toreceive their assigned treatment hasbeen cited as a criticism regarding thereported response rates and survivaldata in these two trials.The first trial to demonstrate a survivaladvantage with HAI comparedto systemic chemotherapy in patientswith unresectable liver metastases waspresented at the American Society ofClinical Oncology (ASCO) AnnualMeeting in 2003. This multi-institutionCancer and Leukemia Group B(CALGB) trial randomized 135 patientsto receive either HAI FUDRplus leucovorin plus dexamethasoneor systemic 5-FU plus leucovorin.Median survival was superior in theHAI group compared to the systemicchemotherapy group (22.7 vs 19.8months, P = .027). Similar to previoustrials, superiority with HAI chemotherapywas also demonstrated incontrol of hepatic metastatic diseaseand overall response rate (48% vs25%, P = .009). Additionally, preliminaryquality-of-life data suggest improvementin the HAI group at 1 yearcompared to the control arm.[25]This CALGB study is encouragingin that it appears that with the safeguardsmentioned above, investigatorsare able to reduce toxicity, thusdemonstrating a statistically significantsurvival benefit and an improvementin quality of life when comparingHAI FUDR to systemic 5-FU. Asmentioned above, the two Europeanstudies used 5-FU with ports and bothstudies had a large number of patientsnot receiving their assigned treatment.Although cited as a criticism, this observationstands out demonstrating that even with highly monitored chemotherapyadministration, this modalityof treatment continues to beplagued by issues regarding its practicalincorporation into standard care.Alternative Strategies
In addition to modifying the dosingregimen of FUDR and adding steroidsand/or leucovorin, an alternativestrategy has been to alternate HAIchemotherapy with systemic chemotherapy.When using strict administrationand dosing guidelines asmentioned above, this approach wouldin theory achieve high local responserates with reduced hepatobiliary complicationswhile simultaneously treatingextrahepatic micrometastases, thusdecreasing the risk for extrahepaticdisease progression. A number ofsmaller phase II studies have investigatedthis concept utilizing a varietyof chemotherapy schedules, most usingsystemic FUDR or 5-FU.[26,27].To date, there have been mixed results,some suggesting improved tolerancewhile others are complicatedby significant systemic and hepatobiliarytoxicity. Currently, systemicoxaliplatin (Eloxatin) and/or irinotecan(Camptosar) are being added toHAI FUDR regimens to achieve thesegoals.[28,29] This theory is yet to beevaluated in a randomized phase IIItrial.HAI as Primary Therapyin Setting of NewChemotherapeutic Options
The above-mentioned CALGB trialdemonstrating improved survivalwith HAI chemotherapy comparedwith systemic 5-FU plus leucovorinbegan enrollment in 1994. Since thattime, newer chemotherapeutic agentshave demonstrated increased activitywith resultant improvement in survivalin the setting of metastaticcolorectal cancer.Currently, with the availability ofirinotecan, oxaliplatin, and 5-FU, mediansurvival for all patients with metastaticcolorectal cancer approachesor exceeds 20 months.[30] No randomizedtrials have been performedcomparing HAI therapy with thesenewer agents. When utilizing thesenewer, more active agents, survival issimilar to the median survival demonstratedin studies using HAI chemotherapyas the primary therapeuticmodality. This brings into questionany survival benefit with HAI comparedto today's standard systemicchemotherapy options. Current investigationinto HAI chemotherapy in thesetting of unresectable hepatic metastasesinvolves combining this modalitywith systemic chemotherapyutilizing these newer, more activeagents,[27] as well as investigatingthese drugs as the primary chemotherapyagent delivered by the hepaticartery pump.[31,32]HAI in the Preoperative SettingWith the remarkably high responserate to HAI chemotherapy, one obviousconsideration is to implement thisapproach in the neoadjuvant setting.One retrospective study at the M. D.Anderson Cancer Center attempted toaddress this question. Over 15 years,383 patients with unresectable livermetastases from colorectal cancerwere treated with HAI chemotherapy.A total of 22 (0.6%) patients underwentexploratory surgery, of which18 received some form of treatment(10 underwent resection, 6 resectionand radiofrequency ablation [RFA] orcryotherapy, and 2 RFA alone). At amedian follow-up of 17 months, 15(83%) of the 18 patients who had receivedtreatment had developed recurrentdisease. The other threepatients had died of other causes within7 months of surgery.[33] Similarly,of over 300 patients at UCSFtreated with HAI chemotherapy, only3 have been explored for surgical resection,without any long-term survivors.[34]There are no prospective randomizedtrials using HAI chemotherapyin the neoadjuvant setting. However,data such as mentioned above havenot been encouraging. Lacking reliableclinical benefit, administeringHAI chemotherapy with a neoadjuvantintent should be used cautiously.Furthermore, the biliary toxicity associatedwith HAI chemotherapy maydiminish hepatic reserve and renderpatients less able to undergo furtherhepatic resections.HAI in the Postoperative SettingInitial interest in HAI chemotherapyas adjuvant therapy following resectionof liver metastases began inthe 1980s. Two studies investigatingthe role of HAI therapy included armsin which patients with resectable solitaryliver lesions were randomized toreceive HAI as adjuvant therapy vsno further therapy. Unfortunately, thestudy designs were complex, involvingmultiple arms; few patients wereenrolled in the adjuvant arms of thesestudies. Meaningful information aboutany potential advantage of HAI asadjuvant chemotherapy was notgained through these studies.[35,36]In 1999, a Japanese retrospectivestudy looked at the effect of HAI therapyin the adjuvant setting after resectionof liver metastases. In thissingle institution, 174 patients had livermetastases resected between 1984and 1988. If patients had normal hepaticarterial anatomy, HAI chemotherapywith 5-FU, aclarubicin, andmitomycin (Mutamycin) was administeredadjuvantly. Their data suggestsa statistically significant benefit withthis therapy, doubling 5-year overallsurvival (20% vs 40%) as well as improving5-year disease-free survival(9% vs 35%).[37]In the past decade, three groupshave specifically looked at the questionof adjuvant HAI therapy afterresection of liver metastases in a randomizedfashion, as summarized inTable 3.
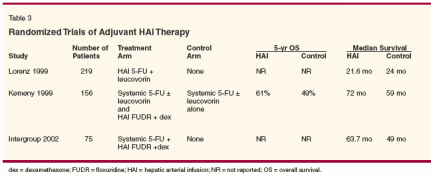
One German study randomized 219patients after resection of liver metastasesto receive either HAI chemotherapywith 5-FU and leucovorin orno further therapy. This study wasterminated early when preliminarydata suggested an improved mediansurvival in the control group comparedto the HAI group (40.8 vs 34.5 months, respectively). There are anumber of criticisms, however. Administering5-FU required the use ofports with its associated complicationsof catheter occlusion and thrombosesas mentioned above. Additionally,with the known lower hepatic extractionof 5-FU, the amount extracted inthis population may be reduced evenfurther after resection of a large portionof the liver. A large proportion ofpatients assigned to the treatment armnever received the assigned therapy(22%) or stopped treatment early(18%). Additionally, the treatmentarm had a significantly higher 30-daypostoperative mortality rate comparedto the control arm for unclear reasons(7.5% vs 2.7%).[38]A study performed at MSKCC randomized156 patients to receive eitherHAI FUDR plus dexamethasonein addition to systemic 5-FU plus leucovorinor to receive the same systemictherapy alone. In this study,median survival (72.2 vs 59.3 months,P = .05) and actuarial 2-year overallsurvival (86% vs 72%, P = .03) favoredthe HAI arm. A trend towardimprovement in 5-year overall survivalis also suggested in the HAI arm(61% vs 49%).[39]Finally, an Intergroup study randomized109 patients to receive eithereitheradjuvant treatment with systemic5-FU and HAI FUDR plus dexamethasoneor no further therapy afterresection of liver metastases. Of the109 patients randomized, 34 patientswere excluded intraoperatively becauseof extrahepatic disease (11 fromthe control arm, 23 from the treatmentarm) limiting the ability to interpretthe results. Nonetheless,intention-to-treat analysis suggesteda nonsignificant poorer median survivalin the treatment arm. However,among treated patients, the 4-year recurrence-free rate (46% vs 25%, P =.04) and 4-year liver recurrence-freerate (67% vs 43%, P = .03) were bothimproved in the treatment arm. Thisstudy also suggested improved survivalin the treatment arm, which didnot reach statistical significance(among treated patients).[40]HAI as Adjuvant Therapyin Setting of NewChemotherapeutic Options
Just as with primary therapy, therole of HAI chemotherapy as adjuvanttherapy must certainly be reevaluatedin the era of newer, moreeffective chemotherapeutic agents.These agents have not been thoroughlyevaluated in this setting. However,the success demonstrated in the recentMOSAIC trial, looking at an oxaliplatin-based regimen in the adjuvantsetting after resection of primary colorectallesions,[41] suggests a possibleimportant role for these neweragents in the adjuvant setting followingresection of hepatic metastaseswhere the risk of recurrence is approximately75%.Similar to the setting of unresectablehepatic metastases, current studiesinvolving HAI chemotherapycombine this modality with systemicchemotherapy utilizing irinotecan oroxaliplatin with 5-FU. Early data fromMSKCC suggests this is a feasibleoption with promising survival data(89% 2-year survival).[42]Conclusions andFuture DirectionsOver the past 25 years, investigationwith HAI chemotherapy has demonstratedthat regionally directed chemotherapy is feasible, and mayresult in significantly improved responserates both as primary therapyand in the adjuvant setting. With improvedpump placement techniquesand rigorous monitoring during drugadministration, HAI chemotherapy-associated toxicity has improved. Yetas recent studies continue to reveal alarge number of patients unable tocomplete the planned HAI chemotherapycourse, the survival benefit usingthis mode of therapy remains uncertain.Additionally, in light of recentprogress in the treatment of metastaticcolorectal cancer using newer chemotherapeuticand other novel agents,the role of HAI chemotherapy aloneas primary or adjuvant therapy appearsto be less clear. However, withhigh clinical response rates and assafety with HAI chemotherapy improves,HAI delivery of these neweragents may prove efficacious. Alternatively,combining this modality ofregional therapy with systemic chemotherapyutilizing newer, more activeagents may prove more effectivethan either modality alone. Phase IItrials evaluating these questions areunder way. Currently, while HAI chemotherapyappears to benefit certainsubsets of patients, its absolute roleremains undefined.
Disclosures:
Dr. Whisenant has nosignificant financial interest or other relationshipwith the manufacturers of any productsor providers of any service mentioned in thisarticle. Dr. Venook receives research supportfrom Roche, Amgen, and Pfizer, and is a consultantfor Genentech and Sanofi.
References:
1.
Kato T, Yasui K, Hirai T, et al: Therapeuticresults for hepatic metastasis of colorectalcancer with special reference to effectivenessof hepatectomy: Analysis of prognostic factorsfor 763 cases recorded at 18 institutions. DisColon Rectum 46:S22-S31, 2003.
2.
Breedis C, Young G: The blood supply ofneoplasms in the liver. Am J Pathol 30:969-985, 1954.
3.
Buchwald H, Grage TB, Vassilopoulos PP,et al: Intraarterial infusion chemotherapy forhepatic carcinoma using a totally implantableinfusion pump. Cancer 45:866-869, 1980.
4.
Habbe T, McCowan T, Goertzen T, et al:Complications and technical limitations of hepaticarterial infusion catheter placement forchemotherapy. J Vasc Interv Radiol 9(2):233-239, 1998.
5.
Ensminger WD, Rosowsky A, Raso V, etal: A clinical-pharmacological evaluation ofhepatic arterial infusions of 5-fluoro-2â²-deoxyuridine and 5-fluorouracil. Cancer Res38:3784-3792, 1978.
6.
Kemeny N, Daly J, Reichman B, et al:Intrahepatic or systemic infusion offluorodeoxyuridine in patients with liver metastasesfrom colorectal carcinoma. Ann InternMed 107(4):459-465, 1987.
7.
Hohn DC, Stagg RJ, Friedman MA, et al:A randomized trial of continuous intravenousversus intraarterial floxuridine in patients withcolorectal cancer metastatic to the liver: TheNorthern Oncology Group Trial. J Clin Oncol7:1646-1654, 1989.
8.
Chang AE, Schneider PD, Sugarbaker PH,et al: A prospective randomized trial of regionalversus systemic continuous 5-fluorodeoxyuridinechemotherapy in the treatment ofcolorectal liver metastases. Ann Surg 206:685-693, 1987.
9.
Martin JK Jr, O’Connell MJ, Wieand HS,et al: Intra-arterial floxuridine vs systemic fluorouracilfor hepatic metastases from colorectalcancer. Arch Surg 125:1022-1027, 1990.
10.
Rougier P, Laplanche A, Huguier M, etal: Hepatic arterial infusion of floxuridine inpatients with liver metastases from colorectalcarcinoma: Long-term results of a prospectiverandomized trial. J Clin Oncol 10(7):1112-1118, 1992.
11.
Allen-Mersh TG, Earlam S, Fordy C, etal: Quality of life and survival with continuoushepatic-artery floxuridine infusion forcolorectal liver metastases. Lancet 344:1255-1260, 1994.
12.
Harmantas A, Rotstein LE, Langer B:Regional versus systemic chemotherapy inthe treatment of colorectal carcinomametastatic to the liver. Cancer 78:1639-1645,1996.
13.
Meta-Analysis Group in Cancer: Reappraisalof hepatic arterial infusion in the treatmentof nonresectable liver metastases fromcolorectal cancer. J Natl Cancer Inst 88(5):252-258, 1996
14.
Campbell CA, Burns RC, Sitzmann JV,et al: Regional chemotherapy devices: Effectof experience and anatomy on complications.J Clin Oncol 11:822-826, 1993.
15.
Hohn DC, Rayner AA, Exonomou JS,et al: Toxicities and complications of implantedpump hepatic arterial and intravenousfloxuridine infusion. Cancer 57:465-470,1986.
16.
Curley SA, Chase JL, Roh MS, et al:Technical considerations and complicationsassociated with the placement of 180 implantablehepatic arterial infusion devices. Surgery114:928-935, 1993.
17.
Stagg RJ, Venook AP, Chase JL, et al:Alternating hepatic intra-arterial floxuridineand fluorouracil: A less toxic regimen for treatmentof liver metastases from colorectal cancer.J Natl Cancer Inst 83:423-428, 1991.
18.
Kemeny N, Seiter K, Niedzwiecki D, etal: A randomized trial of intrahepatic infusionof fluorodeoxyuridine with dexamethasoneversus fluorodeoxyuridine alone in the treatmentof metastatic colorectal cancer. Cancer69:327-334, 1992.
19.
Kemeny N, Seiter K, Conti JA, et al:Hepatic arterial floxuridine and leucovorin forunresectable liver metastases from colorectalcarcinoma. Cancer 73:1134-1142, 1994.
20.
Kemeny N, Conti JA, Cohen A, et al:Phase II study of hepatic arterial floxuridine,leucovorin, and dexamethasone forunresectable liver metastases from colorectalcarcinoma. J Clin Oncol 12:2288-2295, 1994.
21.
Lorenz M, Mueller H: Randomized,multicenter trial of fluorouracil plus leucovorinadministered either via hepatic arterial or intravenousinfusion versus fluorodeoxyuritineadministered via hepatic arterial infusion inpatients with nonresectable liver metastasesfrom colorectal carcinoma. J Clin Oncol18:243-254, 2000.
22.
Kerr DJ, McArdle CS, Ledermann J, etal: Intrahepatic arterial versus intravenous fluorouraciland folinic acid for colorectal cancerliver metastases: A multicentre randomizedtrial. Lancet 361:368-373, 2003
23.
Heinrich S, Petrowsky H, Schwinnen I,et al: Technical complications of continuousintra-arterial chemotherapy with 5-fluorodeoxyuridine and 5-fluorouracil forcolorectal liver metastases. Surgery 133:40-48,2003.
24.
Fordy C, Burke D, Earlam S, et al: Treatmentinterruptions and complications with twocontinuous hepatic artery floxuridine infusionsystems in colorectal liver metastases. Br JCancer 72:1023-1025, 1995.
25.
Kemeny N, Niedzwiecki D, Hollis DR,et al: Hepatic arterial infusion versus systemictherapy for hepatic metastases from colorectalcancer: A CALGB randomized trial of efficacy,quality of life, cost effectiveness, and molecularmarkers. Proc Am Soc Clin Oncol 22:252,2003.
26.
Kemeny N, Conti JA, Sigurdson E, etal: A pilot study of hepatic artery floxuridinecombined with systemic 5-fluorouracil and leucovorin.A potential adjuvant program afterresection of colorectal hepatic metastases. Cancer71:1964-1971, 1993.
27.
Safi F, Bittner R, Roscher R, et al: Regionalchemotherapy for hepatic metastases ofcolorectal carcinoma (continuous intraarterialversus continuous intraarterial/intravenoustherapy). Cancer 64:379-387, 1989.
28.
Kemeny N, Gonen M, Sullivan D, et al:Phase I study of hepatic arterial infusion offloxuridine and dexamethasone with systemicirinotecan for unresectable hepatic metastasesfrom colorectal cancer. J Clin Oncol 19:2687-2695, 2001.
29.
Paty P, Fong Y, Harris R, et al: Updateof phase I studies of hepatic arterial infusionof floxuridine and dexamethasone pous: Systemicoxaliplatin and irinotecan or systemicoxaliplatin snd fluorouracil and leucovorin forunresectable hepatic metastases from colorectalcancer (abstract 1160). Proc Am Soc Clin Oncol22:289, 2003.
30.
Tournigand C, Andre T, Achille E, et al:FOLFIRI followed by FOLFOX6 or the ReverseSequence in Advanced Colorectal Cancer.A randomized GERCOR study. J ClinOncol 22:229-237, 2004.
31.
Fiorentini G, Rossi S, Dentico P, et al:Irinotecan hepatic aterial infusion chemotherapyfor hepatic metastases from colorectalcancer: A phase II clinical study. Tumori89:382-384, 2003.
32.
Boige V, Lacombe S, De Baere T, et al:Hepatic arterial infusion oxaliplatin combinedwith intravenous 5-FU and folinic acid in nonrespectable liver metastasis of colorectal cancer:A promising option for failures to systemicchemotherapy (abstract 1170). Proc Am SocClin Oncol 22:291, 2003.
33.
Meric F, Patt Y, Curley SA, et al: Surgeryafter downstaging of unresectable hepatictumors with intra-arterial chemotherapy. AnnSurg Oncol 7:490-495, 2000.34. Personal communication.
35.
Kemeny MM, Goldberg D, Beatty JD,et al: Results of a prospective randomized trialof continuous regional chemotherapy and hepaticresection as treatment of hepatic metastasesfrom colorectal primaries. Cancer57:492-498, 1986.
36.
Wagman LD, Kemeny MM, Leong L, etal: Prospective, randomized evaluation of thetreatment of colorectal cancer metastatic to theliver. J Clin Oncol 8:1885-1893, 1990.
37.
Ambiru S, Miyazaki M, Ito H, et al:Adjuvant regional chemotherapy after hepaticresection for colorectal metastases. Br J Surg86:1025-1031, 1999.
38.
Lorenz M, Mueller H, Schramm H, etal: Randomized, multicenter trial of surgeryversus surgery followed by adjuvant arterialinfusion with 5-fluorouracil and folinic acid forliver metastases of colorectal cancer. J ClinOncol 228:756-762, 2000.
39.
Kemeny N, Huang Y, Cohen A, et al:Hepatic arterial infusion of chemotherapy afterresection of hepatic metastases fromcolorectal cancer. N Engl J Med 341:2039-2048, 1999.
40.
Kemeny MM, Adak S, Gray B, et al:Combined-modality treatment for resectablemetastatic colorectal carcinoma to the liver:Surgical resection of hepatic metastases in combinationwith continuous infusion of chemotherapy-An Intergroup study. J Clin Oncol20:1499-1505, 2002.
41.
DeGramont A, Banzi M, Navarro M, etal: Oxaliplatin/5FU/leucovorin in adjuvant coloncancer: Results of the international randomizedmosaic trial (abstract 1015). Proc Am SocClin Oncol 22:253, 2003.
42.
Kemeny N, Jarnagin W, Gonen M, et al:Phase I/II study of hepatic arterial therapy withfloxuridine and dexamethasone in combinationwith intravenous irinotecan as adjuvant treatmentafter resection of hepatic metasatases fromcolorectal cancer. J Clin Oncol 21:3303-3309,2003.