Dermatologic Challenges in Cancer Patients and Survivors
The increased approval of anticancer agents has led to unprecedented results, with improved quality of life and longer survival times, resulting in millions of individuals living with a diagnosis of cancer. Whereas these novel medical, surgical, and radiation regimens, or combinations thereof, are largely responsible for these remarkable achievements, a new, unexpected constellation of side effects has emerged. Most notably, cutaneous toxicities have gained considerable attention, due to their high frequency and visibility, the relative effectiveness of anti–skin toxicity interventions, and the otherwise decreasing incidence of systemic or hematopoietic adverse events. Optimal care dictates that dermatologic toxicities must be addressed in a timely and effective fashion, in order to minimize associated physical and psychosocial discomfort, and to ensure consistent antineoplastic therapy. Notwithstanding the critical importance of treatment-related toxicities, dermatologic conditions may also precede, coincide, or follow the diagnosis of cancer. This review provides a basis for the understanding of dermatologic events in the oncology setting, in order to promote attentive care to cutaneous health in cancer patients and survivors.
The increased approval of anticancer agents has led to unprecedented results, with improved quality of life and longer survival times, resulting in millions of individuals living with a diagnosis of cancer. Whereas these novel medical, surgical, and radiation regimens, or combinations thereof, are largely responsible for these remarkable achievements, a new, unexpected constellation of side effects has emerged. Most notably, cutaneous toxicities have gained considerable attention, due to their high frequency and visibility, the relative effectiveness of anti–skin toxicity interventions, and the otherwise decreasing incidence of systemic or hematopoietic adverse events. Optimal care dictates that dermatologic toxicities must be addressed in a timely and effective fashion, in order to minimize associated physical and psychosocial discomfort, and to ensure consistent antineoplastic therapy. Notwithstanding the critical importance of treatment-related toxicities, dermatologic conditions may also precede, coincide, or follow the diagnosis of cancer. This review provides a basis for the understanding of dermatologic events in the oncology setting, in order to promote attentive care to cutaneous health in cancer patients and survivors.
An estimated 1,399,790 cancer diagnoses were made in 2006.[1] Of these, the majority will require interventions with radiation and/or chemotherapy, which, in turn, will contribute to 65% of individuals surviving 5 years after their initial diagnosis. Consequently, the number of cancer survivors has reached considerable numbers-about 9.8 million (or 3.5% of the total population) in the United States.[2,3] This longer survival has underscored the importance of emotional, social, and medical problems as integral components of continued cancer care. Notably, dermatologic issues occur frequently in individuals affected by cancer, leading to significant physical and psychosocial discomfort along with dose modification and/or interruption of important antineoplastic therapy.[4-6]
FIGURE 1

Publications on Dermatologic Toxicities and FDA Approval for Anticancer Drugs
Despite their high frequency and negative impact on quality of life and clinical outcome in some cases, the majority of these untoward events are underrecognized and undertreated. This may be attributed, at least in part, to the recent approval of novel agents with unexpected toxicities, the aforementioned longer survival times, and lack of timely access to dermatology-oncology clinical programs. The importance of this field is underscored by an increasing number of published reports in recent years (Figure 1), as well as the establishment of interdisciplinary clinical programs dedicated to the management of untoward events to cancer therapies.[5] This review describes common and clinically significant dermatologic problems in those whose lives have been affected by cancer.
Skin and the Cancer Patient
FIGURE 2

Dermatologic Events in the Life of the Cancer Patient/Survivor
With dramatic increases in survival rates associated with most cancers[3] and greater use of radiation and novel anticancer agents causing acute and chronic adverse reactions involving the skin,[6] attention has increasingly focused on the dermatologic components of the cancer experience. The skin and its appendages are high-turnover tissues with epithelial, connective tissue, vascular, and neural components-all of which may explain the high frequency of pathobiologic events from drugs directed against rapidly dividing malignant cells. Whereas disorders preceding the diagnosis are of importance, as they may be the first indicators of an underlying neoplasm, and therapy-related events may affect survival, late events can have a significant emotional and physical impact.[4,7,8]
TABLE 1

Malignancy-Associated Dermatoses
Importantly, conditions affecting dermatologic structures (ie, skin, hair, and nails) may present before the diagnosis of cancer, during therapy, or months to years afterwards. The categorization of such events based on chronology (in relation to the initial cancer diagnosis) allows for a better understanding of (1) malignancy-associated dermatoses, which tend to occur prior to or during the initial diagnosis; (2) therapy-related toxicities, which occur during treatment, and; (3) late events, which are usually chronic or persistent sequelae of therapeutic regimens or indicative of recurrence (Figure 2). Knowledge and management of these various manifestations by oncologists would represent a considerable step toward maximizing clinical outcomes and enhancing patients' well-being. This review describes the clinical presentation of common entities and their etiologies in each of these categories, with a greater emphasis on treatment-related events.
Malignancy-Associated Dermatoses
FIGURE 3

Skin Toxicities Associated With Cancer Chemotherapeutic AgentsFIGURE 4
Nail and Hair Toxicities Associated With Cancer Chemotherapeutic Agents
Dermatologic findings prior to or during the diagnosis of cancer may reflect a cancer syndrome that is inherited,[9] caused by environmental carcinogens,[10] or paraneoplastic (Table 1).[11] Whereas the first two entities have well established causal genetic and external culprits, paraneoplastic syndromes are poorly understood, as they represent dermatoses that are distant to an underlying cancer yet believed to be causally and biologically related to it.[11] Overall, the identification of malignancy-associated dermatoses is important, as they may represent the first signs of and allow for earlier diagnosis and treatment of solid or hematologic tumors.[9]
Therapy-Related Dermatologic Toxicities
The clinical presentation and severity of skin toxicities will depend on factors such as dosing, schedule, vehicle, and agent utilized as well as functional status of the patient. Moreover, interactions between anticancer agents should also be taken into consideration, as they may affect drug bioavailability. More than 52 distinct skin toxicities have been reported as a result of 41 unique agents or their combination (Figures 3 and 4).[5]
Cutaneous Reactions
Papulopustular Eruptions
FIGURE 5
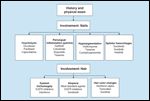
Dermatologic Toxicities Involving Skin
The appearance of erythematous papules or pustules is a common presentation with many agents, most notably those targeting the epidermal growth factor receptor (EGFR). Often inaccurately referred to as acne or acneiform, these toxicities are frequent, occurring in 45% to 100% of patients receiving these drugs (Figure 5A).[5] Interestingly, some studies have shown a relationship between the severity of the rash and tumor response, underscoring the importance of maintaining patients on therapy, as those who could benefit the most are ones who require dose modification.[12]
An inflammatory mechanism underlies this reaction, likely a consequence of altered keratinocyte proliferation, differentiation, migration, and chemokine expression.[5] Early intervention with topical or oral corticosteroids or antibiotics with anti-inflammatory activity (ie, doxycycline, minocycline) has produced improvement in uncontrolled reports.[5,13-15]
Maculopapular/Morbilliform Reaction
An ill-defined, erythematous, mildly pruritic rash especially affecting the upper body is one of the most common reactions to all anticancer agents (Figure 5B). These effects are not considered to be allergic (type I) in nature, as repeated exposures do not lead to greater toxicity.[16-18] Histologic specimens of affected skin will show a mixed inflammatory infiltrate, which, when associated with eosinophils, is suggestive of this phenomenon. True allergic reactions-with anaphylaxis, angioedema, and urticaria-are not as frequent but have been reported with intravenous monoclonal antibodies and other agents, including docetaxel (Taxotere) or paclitaxel.[19] Whereas the more common maculopapular rash responds to topical and/or oral corticosteroids, management of severe reactions consists of premedication with corticosteroids, antihistamines, and acetaminophen.
Blistering Reactions
The impressive clinical presentation of blistering reactions and the consequent urgency that they dictate are well founded-the blistering disorders erythema multiforme, Stevens-Johnsons syndrome (SJS), and toxic epidermal necrolysis (TEN) are fatal in as much as 30% to 40% of cases.[20,21] Erythema multiforme lesions appear as asymptomatic targetoid erythematous dusky round maculopapules with violaceous to dusky centers. SJS syndrome manifests as vesiculobullous lesions with tissue denudation that involve the skin, as well as the conjunctiva, oral, and genital mucosa, affecting < 10% body surface area. TEN has a similar clinical presentation, but involves more than 30% of body surface area.
Treatment is supportive and includes transfer to a burn unit with antihistamines for pruritus, topical lidocaine for oral bullae and erosions, and broad-spectrum antibiotic coverage for possible infections. Discontinuation of the offending agent is mandatory, and the use of intravenous corticosteroids or immunoglobulins remains controversial.[19-22]
Mucositis
Mucositis is a common side effect that may lead to significant morbidity, dose modifications, and life-threatening infections. Radiation, chemotherapy, and conditioning regimens for hematopoietic stem cell transplants are common culprits. Underlying mechanisms are a complex interaction of multiple factors.[23-26] Agents frequently implicated in mucositis are the antimetabolites and antibiotics, and pemetrexed (Alimta), a novel multitargeted antifolate agent that possesses antitumor activity against solid tumors such as mesotheliomas, non–small-cell lung, pancreatic, colorectal, gastric, bladder, breast, and head and neck cancers.[23-26]
Preventive and management strategies for oral mucositis include cryotherapy, and granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine). Benefit may also be obtained from amifostine (Ethyol), chlorhexidine gluconate mouthwashes, benzydamine (Canadian generic name), antibiotic pastilles, and proper oral hygiene. Palifermin (Kepivance) is a recombinant form of human keratinocyte growth factor that stimulates the growth of cells on the surface of the gastrointestinal tract and was shown to decrease the incidence of mucositis and swallowing difficulties. In the case of pemetrexed-associated mucositis, vitamin B12 and folic acid supplementation may reduce most severe toxicities.[23-26]
Hand-Foot Syndrome
FIGURE 6
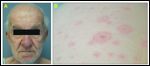
Hand-Foot Syndrome
Another disabling toxicity occurring in 6% to 42% of patients treated with certain agents (such as cytarabine, fluorouracil [5-FU], doxorubicin, and methotrexate), is known as palmoplantar erythrodysesthesia, acral erythema or hand-foot syndrome, and is characterized by the development of erythematous, burning, tender lesions on the palms and soles, with occasional blisters or bullae (Figure 6). This cutaneous toxicity is one of the most common toxicities seen with pegylated liposomal doxorubicin (Doxil), which is doxorubicin encapsulated in polyethylene-coated liposome, and is thought to have a lower risk of cardiac toxicity than the noncoated formulation of doxorubicin.[27,28]
With docetaxel, there is a 70% incidence of cutaneous reactions (such as erythematous to violaceous macules or plaques) in acral locations, usually beginning 2 to 4 days after initiation of therapy, and occasionally causing pruritus or pain.[29] Interference with activities of daily living is frequent, so it is critical to treat these untoward reactions. Histologic examination reveals apoptotic keratinocytes and nonspecific inflammation. Risk factors include higher peak and cumulative doses, age, and female gender.
Therapies include dose modification, pyridoxine, regional cooling, celecoxib (Celebrex), topical urea, and oral corticosteroids.[28] Due to the lack of alternative therapeutic options, these findings have been generalized, so that widespread use of treatments against hand-foot syndrome is recommended for any patient with similar symptoms.
Radiation Dermatitis and Recall
Skin overlying an irradiated site is frequently damaged, which is characterized by erythema, edema, desquamation, pruritus, tenderness, necrosis, and in some cases, ulcers.[30,31] "Recall" occurs when skin overlying previously irradiated sites develops an inflammatory reaction with erythema, vesicles, dermatitis, and desquamation after the administration of antineoplastic agents. This phenomenon occurs from 2 days to 15 years after the initial radiation dose, and may also occur in internal organs, such as the lungs or gastrointestinal system. Data suggest that the late administration of an antineoplastic agent has an effect on tissues defective in stem cells and unable to tolerate an additional insult. Interestingly, higher doses of initial radiation seem to predispose to the development of radiation recall, a phenomenon that may also be secondary to ultraviolet radiation.[30-32]
Intertrigo, Skin Desquamation
Intertrigo appears as pruritic erythematous patches over the axillae, groin, and waist, as well as in high friction areas. It is commonly seen with pegylated liposomal doxorubicin therapy.[33-36] Intertrigo lesions are painful and erosive, and reduction in the drug dose is necessary to alleviate these painful skin lesions. Other treatment options include antifungals if the culprit is Candida species, or low-potency topical steroids. It is important to keep the involved area dry and clean.[34]
Nonspecific erythema and skin desquamation are seen with many chemotherapeutic agents, including alkylating agents, antibiotics, and the camptothecins. Camptothecins exert their antineoplastic activity by binding to the topoisomerase I DNA complex and causing cell death during the S phase of the cell cycle. Two derivatives of camptothecin, irinotecan (Camptosar) and topotecan (Hycamtin), are relatively novel agents used in the treatment of advanced colorectal, gastric, lung, ovarian, and head and neck cancers.[37] Skin erythema and desquamation are seen with camptothecin treatment. Emollients, and ointments, and topical steroids if needed, may help relieve symptoms of pruritus that may be associated with this cutaneous toxicity.[37]
Cutaneous Ulcers and Vasculitic Lesions
Painful leg ulcers, commonly of the lateral malleolus are notoriously associated with hydroxyurea.[38,39] The average onset of ulcers is about 6 years after treatment initiation. Hydroxyurea, a hydroxylated derivative of urea that selectively inhibits DNA synthesis, is used to treat myeloproliferative disorders. Approximately 10% to 35% of patients who are treated with hydroxyurea will manifest skin eruptions. Gemcitabine (Gemzar), cisplatin, and rituximab (Rituxan) have been associated with vasculitis-induced ulceration.[22,40]
Early clinical detection, as manifested by livedoid reticulated pattern of the skin, is essential. In addition, when ulcers occur, a skin biopsy could help distinguish the different etiologies, such as neoplastic, therapeutic, vasculitic or infectious. Management includes discontinuation of the offending agent (hydroxyurea, gemcitabine), and prednisone at 1 mg/kg/d.[40] Appropriate ulcer care is critical for patient comfort and care.
Hyperpigmentation
The antimetabolites, especially 5-FU, are known to cause reticulate hyperpigmentation. Hyperpigmentation and melanonychia have been reported to occur in 24% of patients treated with 5-FU. When infused intravenously, 5-FU causes a brown hyperpigmentation of the supravenous skin in 2% to 5% of patients. This hyperpigmentation may persist after discontinuing the drug and may increase in intensity after sun exposure. Eruptive pigmented skin lesions including lentigo maligna–like lesions are also known to occur with 5-FU treatment.[40] Dactinomycin (Cosmegen) has been associated with an erythematous linear pigmentation with follicular prominence and central desquamation.[40]
Hyperpigmentation-global, localized, or the so-called flagellate-is also known to occur in patients treated with bleomycin. Flagellate hyperpigmentation refers to linear streaks of hyperpigmentation, and these streaks are known to occur after a cumulative dose ranging between 90 and 285 mg.[40,41] They can manifest as early as 24 hours or as late as 9 weeks after initiation of bleomycin therapy. As many as 8% to 38% of patients on bleomycin develop these streaks. Fortunately, the hyperpigmentation is reversible after discontinuation of bleomycin.[40,41]
Inflammation of Actinic Keratoses
Inflammation of actinic keratoses occurs with cytarabine, capecitabine (Xeloda), gemcitabine, doxorubicin, bleomycin, vincristine, and 5-FU.[42,43] Actinic (solar) keratoses are highly common erythematous macules with fine gritty scale that are thought to be precursors to squamous cell carcinoma of the skin. When they get inflamed, actinic keratoses become bright red, pruritic, and sometimes painful. It is not surprising that inflammation of actinic keratoses occurs with cytarabine and 5-FU, since topical 5-FU is used to treat these lesions. Therefore, this side effect of 5-FU and cytarabine is considered to be beneficial, and treatment should not be discontinued. Topical steroids can be used to alleviate the inflammation.[42,43]
Connective Tissue Abnormalities
A dermatomyositis-like eruption was observed in patients on long-term treatment with hydroxyurea for myeloproliferative conditions. Such eruption manifests as violaceous erythematous papules over the dorsal metacarpal and interphalangeal joints of the hands (Gottron's papules), as well as periungual telangiectasias and erythema, and cuticular dystrophy.[38] Scleroderma-like reactions were reported to occur in patients receiving taxanes. Docetaxel and paclitaxel are considered among the most potent chemotherapeutic agents that can be used to target multiple solid tumors, especially advanced breast cancer.[44] Their mechanism of action involves the disruption of microtubules, hence, mitosis. Scleroderma-like skin changes include skin tightening of the upper and lower extremities, as well as contracture of the knees and all finger joints. In some cases these fibrotic changes are reversible upon discontinuation of the drug.[44]
Skin Atrophy
5-FU induces skin atrophy that could persist even after discontinuation of the drug, because it inhibits fibroblast proliferation. Collagen, hyaluronic acid, or other fillers can be used to manage such atrophy if it occurs in noticeable areas.[45] In one study, 13% of patients on long-term hydroxyurea developed mucocutaneous atrophy, usually on the legs and other sun-exposed sites.[39]
Hair Alterations
FIGURE 7
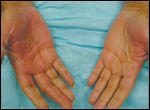
Hair Alterations
Alopecia is one of the most well-known dermatologic alterations due to cancer therapies (Figure 7A), and although it does not have a significant impact on physical health, it does lead to decreased quality of life.[46] Hair loss is usually reversible, although the hair may grow with different texture.
Figure 3 lists the most common chemotherapeutic agents that cause hair abnormalities including alopecia. These agents include but are not limited to doxorubicin, dactinomycin, cytarabine, and etoposide (60% of patients experience hair alterations). For instance, cytarabine is associated with alopecia within 2 to 4 weeks of the initiation of therapy.
Cooling the scalp before, during, and after chemotherapy decreases the incidence of alopecia.[47-49] Other modalities used to decrease scalp alopecia include scalp tourniquets and minoxidil. Tourniquet pressure is thought to decrease scalp blood flow.[49-51] Longer and thicker eyelashes (trichomegaly) develop in up to 33% of patients receiving EGFR inhibitors (Figure 7B).
Nail and Periungual Reactions
FIGURE 8
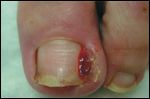
Nail Alterations
Periungual inflammation is one side effect that causes significant pain. It is characterized by paronychial edema, erythema, and tenderness as well as onycholysis on the affected fingers or toes (Figure 8). Although not usually responsive to antistaphylococcal therapy, minocycline, doxycycline, and topical steroids have been used to treat paronychial inflammation.[13,52]
The EGFR inhibitors have been implicated in paronychial inflammation. Cetuximab (Erbitux), a chimeric immunoglobulin (Ig)G1 monoclonal antibody to the EGFR receptor, has been reported to induce-in addition to the papulopustular eruption on the face, chest, and upper back-follicular plugging, neutrophilic folliculitis, and tender paronychial inflammation of toes and fingers.[52]
Onycholysis and onychomadesis have both been reported as cutaneous side effects of capecitabine, bleomycin, and hydroxyurea.[53] With hydroxyurea, nail changes are less common and include brittle nails, onychodystrophy, onycholysis, and melanonychia (longitudinal more than transverse).[40]
Docetaxel, a taxane, induces onycholysis, and dyschromia of the nails, subungual hematoma and suppuration, acute paronychia, and Beau's lines. The incidence of such nail changes ranges from 0% to 44%.[54] A frozen glove was demonstrated to prevent such nail changes, when worn by patients 15 minutes before the administration of docetaxel, during the 1-hour docetaxel infusion, and 15 minutes after the end of infusion.[54]
Late Events
Dermatologic entities in cancer survivors after completion of scheduled regimens range from therapy-induced sequelae to signs indicating recurrent disease. Although this category of dermatoses remains the most underreported and underrecognized, it is anticipated that increased survival times with newer agents, and consequent attention on survivorship issues will bring about further interest in this topic.
Scars
Scarring associated with oncologic surgical procedures has been associated with psychological problems in 15% to 16% of survivors of childhood cancer.[4] Both short- and long-term psychological repercussions have been found in breast cancer survivors who received mastectomies, especially in younger women. Not surprisingly, patients with disfiguring skin conditions with a prior diagnosis of head and neck squamous cell carcinoma had significant psychosocial difficulties, with high levels of anxiety, depression, and social anxiety and avoidance, and decreased quality of life.
Chronic Radiation Dermatitis
Chronic radiation dermatitis changes develop months to years after radiation exposure. These changes can include hyper- or hypopigmentation, scaling, xerosis, and thickened or hyperkeratotic skin. In addition, irradiated areas are devoid of hair follicles or sebaceous glands, yet have prominent blood vessels manifested as telangiectasias. Treatment is symptomatic and includes different modalities such as pulsed dye laser for telangiectasia, hyperbaric oxygen therapy to alleviate the pain caused by edema, erythema, or lymphedema, as well as keratolytic agents for the scaling and xerosis.[55]
Increased Secondary Cancers
Therapy-related skin cancers, especially basal cell carcinoma, have been described in survivors of childhood and adolescent cancer. Such cancers are known as second primary cancers, and they could be the result of factors that caused the primary cancer in the first place, such as smoking, drinking alcohol, alterations in hormonal and immunologic status, and environmental factors. Other factors also include the carcinogenic effects of cytotoxic drugs and radiation therapy.[56]
It is well established that ionizing radiation increases the likelihood of precancerous and cancerous skin lesions such as basal and squamous cell carcinomas. This is attributed to the fact that irradiated cells are genetically unstable, therefore more prone to mutations. In addition, cancer patients may have mutations in their tumor-suppressor genes; hence, they might have an increased risk of other malignancies. Although other skin malignancies such as melanomas and angiosarcomas are thought to be radiation-related, this conclusion cannot be drawn with certainty due to the lack of definitive data.[57]
Cutaneous Metastases
Cutaneous metastases are a frequent sign of recurrence but may be overlooked, as their appearance tends to be unimpressive.[58-60] Although uncommon, cutaneous metastases are often a sign of advanced grade and poor prognosis. The most common malignancies associated with metastases to the skin are breast and lung cancer, and the most common site of cutaneous metastasis is the chest.[58-61] Therefore, clinicians should have a high level of suspicion when cancer survivors present with the sudden onset of nodules or firm papules that are persistent, specifically if they are located on the chest.
Other cancers that have been reported to metastasize to the skin include colorectal, stomach, ovarian, bladder, and renal cancers.[58-62] A rare form of cutaneous metastasis from breast cancer is a form of scarring alopecia, known as alopecia neoplastica.[63,64] Lung cancer, especially adenocarcinoma, has been known to metastasize to the skin, which correlates with a poor prognosis.
The most common sites for metastases include the chest and abdominal walls. However, other areas such as the lip, scrotum, and perianal skin have been described.[58-60] Renal cell carcinoma has been reported to metastasize to the skin, presenting as nodules to the scalp (cutaneous horn or pyoderma gangrenosum–like) or to the subungual unit.[58-60] In ovarian cancer, the most common cutaneous metastasis is known as Sister Joseph's nodule, a rare cutaneous metastasis to the umbilicus.[62] Clinically, it is a painful, sometimes ulcerated nodule with irregular borders that is firmly attached to the anterior abdominal wall. A bloody, mucinous, or purulent discharge may be present. The size of this nodule is as variable as its morphology, and it has been reported to be as large as 10 cm.[64]
Graft-vs-Host Disease
Stem cell transplantation has proven to be lifesaving in many malignancies. The triad of dermatitis, hepatitis, and enteritis arising within 100 days after transplantation is described as acute graft-vs-host disease (GVHD).[65,66] Classically, acute cutaneous GVHD occurs 2 to 3 weeks after transplants, and it manifests as a pruritic or painful rash. The rash is initially localized to the palms and soles, and gradually involves the entire body, occasionally with bullae and vesicle formation and erythroderma. In both acute and chronic GVHD (that which appears after 100 days), xerosis is an extremely common complaint, and proper management with hydrating agents and soft gentle soaps is essential for patient comfort.
Other cutaneous manifestations of GVHD include diffuse alopecia, and sclerodermoid skin changes. Mucosal changes include oral mucosa atrophy, erosions, and ulceration, pyogenic granulomas, xerostomia, oral lichen planus–like changes, and submucosal fibrosis. Nail changes are also common, presenting as longitudinal streaks and roughness of the nail plates. It is essential to be vigilant and detect these skin changes, as progression to other organs could be fatal.[65,66]
Summary
The success of improved detection and therapeutic strategies has led to a remarkable 65% survival rate at 5 years for all cancers.[1-3] As such, issues relating to the emotional and physical well-being of cancer survivors have gained increased attention. Importantly, unintended dermatologic consequences of surgery, chemotherapy, or radiation are common in treated individuals, all of which may have a significant impact on quality of life and physical status.
Although dermatologic care for cancer patients through the establishment of specialized dermatology clinics will contribute to better knowledge and treatment options, it still remains a damage control exercise that focuses on directly addressing urgent issues so that patients may continue on antineoplastic therapy. Progress in dermatologic care for cancer patients commands increased attention to the skin and appendages as part of everyday patient care and during clinical trials. Moving forward, careful observation and insightful analysis between dermatologists and oncologists will generate recommendations for new and improved dermatologic care in oncology.
Financial Disclosure: Drs. Agha and Bennettand Ms. Kinahan have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article. Dr. Lacouture is a speaker for ImClone and Onyx and a consultant for Bristol-Myers Squibb and Bayer.
References:
1. American Cancer Society: Cancer Facts & Figures 2006. Available at www.cancer.org. Accessed August 29, 2007.
2. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2006. CA Cancer J Clin 56:106-130, 2006.
3. Centers for Disease Control and Prevention: Cancer survivorship-United States, 1971-2001. MMWR Morb Mortal Wkly Rep 53:526-529, 2004.
4. Pinter AB, Hock A, Kajtar P, et al: Long-term follow-up of cancer in neonates and infants: A national survey of 142 patients. Pediatr Surg Int 19:233-239, 2003, 2003.
5. Lacouture ME, Basti S, Patel J, et al: The SERIES clinic: An interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol 4:236-238, 2006.
6. Robert C, Soria JC, Spatz A, et al: Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6:491-500, 2005.
7. Molinari E, De Quatrebarbes J, Andre T, et al: Cetuximab induced acne. Dermatology 211:330-333, 2005.
8. Kurzrock R: Cutaneous paraneoplastic syndromes in solid tumors. Am J Med 99:662-671, 1995.
9. Tsao H: Update on familial cancer syndromes and the skin. J Am Acad Dermatol 42:939-969, 2000.
10. Kurzrock R, Cohen PR: Mucocutaneous paraneoplastic manifestations of hematologic malignancies. Am J Med 99:207-216, 1995.
11. Badawi RA, Birns J: Acanthosis nigricans associated with acute myeloid leukaemia. Eur J Int Med 15:473, 2004.
12. Perez-Soler R: Can rash associated with HER1/EGFR inhibition be used as a marker of treatment outcome? Oncology (Williston Park) 17(11 suppl 12):23-28, 2003.
13. Shu KY, Kindler HL, Medenica M, et al: Doxycycline for the treatment of paronychia induced by the epidermal growth factor receptor inhibitor cetuximab. Br J Dermatol 154:191-192, 2006.
14. Perez-Soler R, Saltz L: Cutaneous adverse effects with HER1/EGFR-targeted agents: Is there a silver lining? J Clin Oncol 23:5235-5246, 2005.
15. Lee MW, Seo CW, Kim SW, et al: Cutaneous side effects in non-small cell lung cancer patients treated with Iressa (ZD1839), an inhibitor of epidermal growth factor. Acta Derm Venereol 84:23-26, 2004.
16. Ayangco L, Rogers RS 3rd: Oral manifestations of erythema multiforme. Dermatol Clin 21:195-205, 2003.
17. Habif TP: Clinical Dermatology: A Color Guide to Diagnosis and Therapy, 4th ed. St. Louis, CV Mosby, 2003.
18. Lo SK, Yip D, Leslie M, et al: 5-fluorouracil-induced erythema multiforme. Int J Clin Pract 53:219-221, 1999.
19. Lowndes S, Darby A, Mead G, et al: Stevens-Johnson syndrome after treatment with rituximab Ann Oncol 13:1948-1950, 2002.
20. Hellier I, Bessis D, Sotto A, et al: High-dose methotrexate-induced bullous variant of acral erythema. Arch Dermatol 132:590-591, 1996.
21. Cakesen H, Oner AF: Toxic epidermal necrolysis in a girl with leukemia receiving methotrexate. Indian Pediatr 38:426, 2001.
22. Scheinfeld NA: Review of rituximab in cutaneous medicine. Dermatol Online J 12:3, 2006.
23. Cetkovská P, Pizinger K, Cetkovský P: High-dose cytosine arabinoside-induced cutaneous reactions J Eur Acad Dermatol Venereol 16:481-485, 2002.
24. Horsley P, Bauer JD, Mazkowiack R, et al: Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation Support Care Cancer 15:105-109, 2007.
25. Pereira Pinto L, de Souza LB, Gordon-Nunez MA, et al: Prevention of oral lesions in children with acute lymphoblastic leukemia. Int J Pediatr Otorhinolaryngol 70:1847-1851, 2006.
26. Djuric M, Hillier-Kolarov V, Belic A, et al: Mucositis prevention by improved dental care in acute leukemia patients. Support Care Cancer 14:137-146, 2006.
27. Banfield GK, Crate ID, Griffiths CL: Long-term sequelae of palmar-plantar erythrodysaesthesia syndrome secondary to 5-fluorouracil therapy. J R Soc Med 88:356P-357P, 1995.
28. Vukelja SJ, Baker WJ, Burris HA 3rd, et al: Pyridoxine therapy for palmar-plantar erythrodysesthesia associated with taxotere. J Natl Cancer Inst 85:1432-1433, 1993.
29. Zimmerman GC, Keeling JH, Burris HA, et al: Acute cutaneous reactions to docetaxel, a new chemotherapeutic agent. Arch Dermatol 131:202-206, 1995.
30. Jeter MD, Jänne PA, Brooks S, et al: Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys 53:394-400, 2002.
31. Hureaux J, Le Guen Y, Tuchais C, et al: Radiation recall dermatitis with pemetrexed. Lung Cancer 50:255-258, 2005.
32. Borroni G, Vassallo C, Brazzelli V, et al: Radiation recall dermatitis, panniculitis, and myositis following cyclophosphamide therapy: Histopathologic findings of a patient affected by multiple myeloma. Am J Dermatopathol 26:213-216, 2004.
33. Chou HH, Wang KL, Chen CA, et al, for the Taiwanese Gynecologic Oncology Group: Pegylated liposomal doxorubicin (Lipo-Dox) for platinum-resistant or refractory epithelial ovarian carcinoma: A Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol Oncol 101:423-428, 2006.
34. English JC 3rd, Toney R, Patterson JW: Intertriginous epidermal dysmaturation from pegylated liposomal doxorubicin. J Cutan Pathol 30:591-595, 2003.
35. Kim RJ, Peterson G, Kulp B, et al: Skin toxicity associated with pegylated liposomal doxorubicin (40 mg/m2) in the treatment of gynecologic cancers. Gynecol Oncol 97:374-378, 2005.
36. Lotem M, Hubert A, Lyass O, et al: Skin toxic effects of polyethylene glycol–coated liposomal doxorubicin. Arch Dermatol 136:1475-1480, 2000.
37. Pizzolato JF, Saltz LB: The camptothecins. Lancet. 361:2235-2242, 2003.
38. Varma S, Lanigan SW: Dermatomyositis-like eruption and leg ulceration caused by hydroxyurea in a patient with psoriasis. Clin Exp Dermatol 24:164-166, 1999.
39. Vassallo C, Passamonti F, Merante S, et al: Muco-cutaneous changes during long-term therapy with hydroxyurea in chronic myeloid leukaemia. Clin Exp Dermatol 26:141-148, 2001.
40. Wyatt AJ, Leonard GD, Sachs DL: Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol 7:45-63, 2006.
41. Kumar Rajesh, Pai Vishaka: Bleomycin induced flagellate pigmentation. Indian Pediatr 43:74-75, 2006.
42. Ilyas EN, Grana G, Green JJ: Inflammatory actinic keratoses secondary to systemic chemotherapy. Cutis 75:167-168, 2005.
43. Lewis KG, Lewis MD, Robinson-Bostom L, et al: Inflammation of actinic keratoses during capecitabine therapy. Arch Dermatol 140:367-368, 2004.
44. Cleveland MG, Ajaikumar BS, Reganti R: Cutaneous fibrosis induced by docetaxel: A case report. Cancer 88:1078-1081, 2000.
45. Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, et al: Intralesional 5-fluorouracil in the treatment of keloids: An open clinical and histopathologic study. J Am Acad Dermatol 52:474-479, 2005.
46. Remlinger KA: Cutaneous reactions to chemotherapy drugs. The art of consultation. Arch Dermatol 139:77-81, 2003.
47. Katsimbri P, Bamias A, Pavlidis N: Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer 36:766-771, 2000.
48. Lemenager M, Lecomte S, Bonneterre ME, et al: Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer 33:297-300, 1997.
49. Ridderheim M, Bjurberg M, Gustavsson A: Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: A pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer 11:371-377, 2003.
50. Hussein AM: Protection against cytosine arabinoside-induced alopecia by minoxidil in a rat animal model. Int J Dermatol 34:470-473, 1995.
51. Hussein AM: Chemotherapy-induced alopecia: new developments. South Med J 86:489-496, 1993.
52. Busam KJ, Capodieci P, Motzer R, et al: Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C22553. Br J Dermatol 144:1169-1176, 2001.
53. Muñoz A, Barceló R, Rubio I, et al: Onycholysis associated with capecitabine in combination with irinotecan in two patients with colorectal cancer. J Natl Cancer Inst 95:1252-1213, 2003.
54. Scotté F, Tourani J-M, Banu E, et al: Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol 23:4424-4429, 2005.
55. Ruiz-Genao DP, Córdoba S, GarcÃa-F-Villalta MJ, et al: Post-radiotherapy telangiectasias. Treatment with pulsed-dye laser. Sequential histological studies. Actas Dermosifiliogr 97:345-347, 2006.
56. Fogarty GB, Bayne M, Bedford P, et al: Three cases of activation of cutaneous squamous-cell carcinoma during treatment with prolonged administration of rituximab. Clin Oncol (R Coll Radiol) 18:155-156, 2006.
57. Salmon-Ehr V, Grosieux C, Potron G, et al: Multiple actinic keratosis and skin tumors secondary to hydroxyurea treatment. Dermatology 196:274, 1998.
58. Perng DW, Chen CH, Lee YC, et al: Cutaneous metastasis of lung cancer: An ominous prognostic sign. Zhonghua Yi Xue Za Zhi (Taipei) 57:343-347, 1996.
59. Peterson JL, McMarlin SL: Metastatic renal cell carcinoma presenting as a cutaneous horn. J Dermatol Surg Oncol 9:815-818, 1983.
60. Cohen PR: Metastatic tumors to the nail unit: Subungual metastases. Dermatol Surg 27:280-293, 2001.
61. Damin DC, Lazzaron AR, Tarta C, et al: Massive zosteriform cutaneous metastasis from rectal carcinoma. Tech Coloproctol 7:105-107, 2003.
62. Gabriele R, Borghese M, Conte M, et al: Sister Mary Joseph's nodule as a first sign of cancer of the cecum: Report of a case. Dis Colon Rectum 47:115-117, 2004.
63. Baum EM, Omura EF, Payne RR, et al: Alopecia neoplastica-a rare form of cutaneous metastasis. J Am Acad Dermatol 4:688-694, 1981.
64. Lin WL, Lin WC, Jung SM, et al: Breast cancer metastasized to the scalp mimicking alopecia areata: Alopecia neoplastica. Breast J 13:94-95, 2007.
65. Sanli H, Ekmekçi P, Arat M, et al: Clinical manifestations of cutaneous graft-versus-host disease after allogeneic haematopoietic cell transplantation: long-term follow-up results in a single Turkish centre. Acta Derm Venereol 84:296-301, 2004.
66. Huang J, Pol-Rodriguez M, Silvers D, et al: Acquired ichthyosis as a manifestation of acute cutaneous graft-versus-host disease. Pediatr Dermatol 24:49-52, 2007.