Liposomal Doxorubicin in Combination With Bortezomib for Relapsed or Refractory Multiple Myeloma
Liposomal doxorubicin received FDA approval for use in combination with bortezomib in patients with multiple myeloma who have not previously received bortezomib and have received at least one prior therapy.
Purpose: On May 17, 2007, doxorubicin HCl liposome injection (Doxil) in combination with bortezomib (Velcade) received approval from the US Food and Drug Administration (FDA) for the treatment of relapsed or refractory multiple myeloma after at least one prior therapy that has not included bortezomib. Liposomal doxorubicin's efficacy and safety were demonstrated in a phase III, randomized, multicenter, international trial comparing the combination of this agent plus bortezomib vs bortezomib alone in multiple myeloma patients who had not previously received bortezomib and had received at least one prior therapy. Here we summarize the FDA review of the data that support this approval.
Experimental Design and Results: An interim analysis of time to disease progression (TTP), the primary end point, was conducted after 249 TTP events in this study that randomized 324 patients to liposomal doxorubicin plus bortezomib treatment and 322 patients to bortezomib monotherapy. Time to progression was significantly prolonged in the combination arm (median TTP = 9.3 months) compared with bortezomib monotherapy (median TTP = 6.5 months), P < .0001 (log-rank test); hazard ratio = 0.55 (95% confidence interval = 0.43–0.71). The response rates were similar between the two arms and not statistically different; however, among responding patients, the median duration of response was longer with the combination-10.2 months compared to 7.0 months in the monotherapy arm. Adverse reactions occurred more frequently with the combination therapy. As compared to the monotherapy, frequent grade 3/4 adverse reactions with the combination were neutropenia and thrombocytopenia.
Conclusions: Liposomal doxorubicin received FDA approval for use in combination with bortezomib in patients with multiple myeloma who have not previously received bortezomib and have received at least one prior therapy.
Doxorubicin HCl liposome injection (Doxil) received accelerated approval in November 1995 for the treatment of AIDS-related Kaposi's sarcoma in patients with disease that has progressed on prior combination chemotherapy or in patients who are intolerant to such therapy. In June 1999, the drug received accelerated approval for the treatment of metastatic carcinoma of the ovary in patients with disease that is refractory to both paclitaxel- and platinum-based chemotherapy regimens, which was followed by regular approval in January 2005 for the treatment of patients with ovarian cancer whose disease has progressed or recurred after platinum-based chemotherapy.
Relapsed/refractory multiple myeloma constitutes a major challenge for both patients and oncologists.[1,2] Therapeutic options for this disorder include retreatment with initial chemotherapy regimens such as vincristine, doxorubicin, and dexamethasone, thalidomide (Thalomid), bortezomib (Velcade), lenalidomide (Revlimid), and autologous or allogeneic hematopoietic cell transplantation. With thalidomide accepted as front-line therapy for a number of patients, bortezomib and lenalidomide have become treatment options for relapsed or refractory disease.
In a randomized, open-label phase III trial in patients with multiple myeloma previously treated with at least one prior therapy, patients receiving bortezomib alone demonstrated a median time to progression of 6.2 vs 3.5 months with dexamethasone.[3,4] In addition, two randomized, double-blind, placebo-controlled phase III trials showed that the combination of lenalidomide with dexamethasone was associated with a prolonged time to progression compared with placebo plus dexamethasone in patients with multiple myeloma who had received at least one prior therapy.[5,6] Various combinations have been studied incorporating bortezomib or lenalidomide with the aim of enhancing efficacy.[1,7] The study supporting this supplemental new drug application (sNDA) combined pegylated liposomal doxorubicin with bortezomib to treat patients with relapsed and/or refractory multiple myeloma who have received at least one prior therapy.
The protocol for this study in patients with progressive multiple myeloma was submitted and reviewed by the US Food and Drug Administration (FDA) prior to its initiation. The protocol was activated in August 2004. In December 2004, liposomal doxorubicin received orphan drug designation for the treatment of patients with multiple myeloma whose disease has progressed after at least one prior therapy or was refractory to initial therapy. In November 2006, the sNDA was submitted and received a designation for priority review (resulting in a 6-month review timeline). Here we summarize the FDA analysis of the study results.
Study Design for DOXIL-MMY-3001
This liposomal doxorubicin sNDA was based primarily on the results of the DOXIL-MMY-3001 study, an open-label, randomized, multicenter international trial of liposomal doxorubicin plus bortezomib vs bortezomib alone in patients with relapsed or refractory multiple myeloma who have had at least one prior therapy. Prior to randomization, patients were stratified based on beta2-microglobulin level (≤ 2.5 mg/L; > 2.5 mg/L and ≤ 5.5 mg/L; or > 5.5 mg/L) and their response to prior treatment (relapsed vs refractory). Randomization was in a 1:1 allocation within each stratum to the two treatment arms.
Inclusion criteria mainly included the following: (1) patients with confirmed diagnosis of multiple myeloma that was measurable as determined by monoclonal protein in serum (> 1g/dL) or urine (> 200 mg/24 h); (2) progressive disease or primary refractory disease following at least one prior therapy, as defined by > 25% increase in monoclonal protein or development of new or worsening lytic bone lesions, plasmacytoma, or hypercalcemia; (3) adequate bone marrow, renal, and hepatic function as reflected by baseline creatinine clearance ≥ 30 mL/min, aspartate aminotransferase and alanine aminotransferase ≤ 2.5 × upper limit of normal, absolute neutrophil count ≥ 1.00 × 109/L, platelet count ≥ 75 × 109/L, hemoglobin ≥ 8.0 g/dL, and serum calcium, corrected < 12 mg/dL or ionized calcium < 6.5 mg/dL; (4) left-ventricular ejection fraction (LVEF) within institutional normal limits; and (5) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1 with a life expectancy of at least 3 months.
Exclusion criteria are summarized as follows: (1) history of treatment with bortezomib; (2) prior treatment with doxorubicin or other anthracycline at cumulative doses greater than 240 mg/m2; (3) nonsecretory or nonmeasurable disease; (4) progressive disease while receiving an anthracycline-containing regimen or no change in disease status during initial therapy; (5) peripheral neuropathy of grade 2 or higher severity; (6) cardiac conditions such as myocardial infarct within 6 months before enrollment, symptomatic heart failure, or uncontrolled angina; (7) treatment with other chemotherapeutics, radiation therapy, or major surgery within 21 to 30 days before randomization; (8) seropositive for human immunodeficiency virus, or active hepatitis A, B, or C infection; or (9) other poorly controlled medical conditions that could interfere with adherence to or completion of the study.
The primary efficacy endpoint was time to progression (TTP), defined as the interval between the date of randomization and the date of disease progression or death due to progression. The date of disease progression was determined as the date of the first indication of progression. Patients who were progression-free (including those who died without documented progression) at the time of data cutoff were censored for TTP at the time of their last tumor assessment. Disease progression was assessed using the European Group for Blood and Marrow Transplantation (EBMT) criteria established in 1998 and confirmed by an independent data-management committee (IDMC). Secondary endpoints included overall survival, response rate, and duration of response.
The study was designed to detect an improvement in median TTP from 6 months with bortezomib monotherapy to 7.8 months with combination therapy. With 80% power and an overall significance level of 5% (two-sided), the estimated sample size was to have approximately 630 subjects (315 per treatment arm) randomized to observe 460 events (progression or death due to progression). An interim analysis of TTP was planned after approximately 230 events were observed. The collection of TTP data was planned to stop should the data strongly favor treatment with the combination liposomal doxorubicin plus bortezomib as compared to bortezomib alone.
Patients assigned to the combination therapy were to receive liposomal doxorubicin every 3 weeks at a dose of 30 mg/m2 as a 1-hour intravenous infusion on day 4 following bortezomib. Bortezomib at 1.3 mg/m2 was administrated in both arms as an intravenous bolus on days 1, 4, 8, and 11, every 3 weeks. Treatment continued until disease progression, ≥ 14 days delay in starting a new cycle, the occurrence of unacceptable treatment-related toxicity, or for up to a total of eight cycles of therapy, with the exception of a continuing decrease in monoclonal protein > 25% from course to course.
For bortezomib-related neuropathy, dose modifications followed the bortezomib label; for liposomal doxorubicin–related hand-foot syndrome and stomatitis, dose modifications followed the liposomal doxorubicin label.[8] For cardiac toxicities, doxorubicin was supposed to be discontinued if patients experienced symptomatic arrhythmia, congestive heart failure, or an absolute decrease ≥ 15% in LVEF, or LVEF decrease to less than an institution's lower normal limit and absolute decrease ≥ 5%.
Patients underwent clinical and laboratory evaluations every 3 weeks for the first 42 weeks, then every 6 weeks until progression. Serum and urine protein measurements were assayed in a central laboratory, and the results were used by all investigators for assessing response.
Patients were permitted to receive supportive therapies such as bisphosphonates, hematopoietic cytokines (not used prophylactically), blood product transfusions, and antiemetics.
Study Results
The trial randomized 646 patients with progressive multiple myeloma between December 2004 and March 2006 in 123 study centers from 18 countries; 322 were assigned to the bortezomib arm and 324 to the liposomal doxorubicin–plus-bortezomib arm. These 646 patients comprised the intent-to-treat (ITT) population. Of 646 patients, 636 had at least one treatment after their randomization, and were thus included in the population for safety analysis.
Demographics
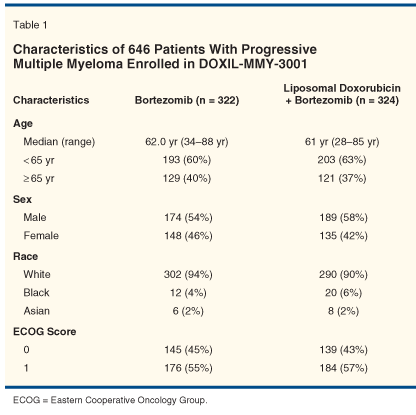
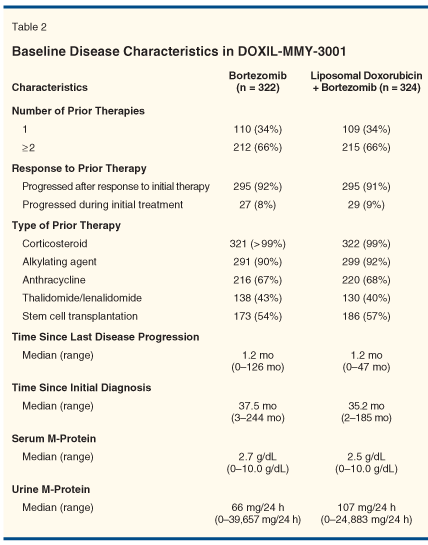
The patient characteristics and disease status at enrollment were balanced between the two treatment arms, as shown in Tables 1 and 2. Most patients in both arms experienced disease progression after their response to initial therapy, with some primarily refractory to initial therapy. Two-thirds of patients had more than one line of prior therapy. Notably, two-thirds of patients also had prior treatment with anthracyclines. Disease burden as reflected by the levels of monoclonal proteins appeared similar between the treatment arms as well.
Protocol violations and deviations were similar between the two arms. The major violations and/or deviations that may have affected efficacy assessment were enrollment of patients who did not meet the criteria of measurable disease and use of protocol-contraindicated radiotherapy, other chemotherapeutics, or steroids during the trial. These departures represented 7% of patients in the combination arm and 5% in the bortezomib arm. Sensitivity analyses with exclusion of those patients did not alter the TTP results.
Treatment intensity and duration appeared to be similar between the two arms, with a median number of treatment cycles of five for each arm at the time of interim analysis. The median cumulative dose of liposomal doxorubicin was 147 mg/m2.
Efficacy
The interim analysis of TTP (as prespecified after approximately 230 events) in the ITT population was performed in August 2006 with 249 events (clinical data cutoff: April 28, 2006). The result favored the combination therapy. With the IDMC recommendation, the collection of TTP data ceased and the protocol was amended in September 2006 to allow patients who were actively on bortezomib monotherapy to cross over to the liposomal doxorubicin/bortezomib combination therapy. As shown in Table 3, the results for TTP, response rate, and duration of response were based on the interim analysis of the ITT population.
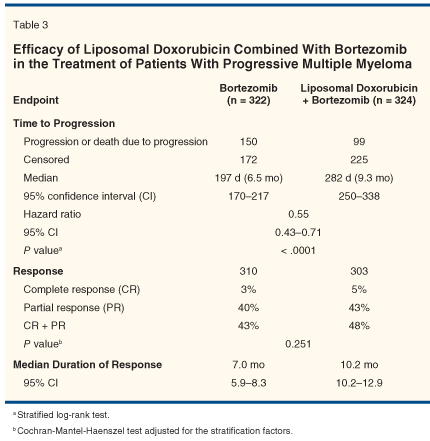
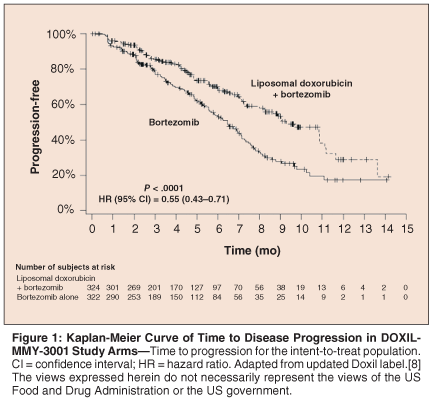
The primary endpoint of TTP was improved significantly in patients treated with liposomal doxorubicin/bortezomib combination therapy (median = 9.3 months) as compared to bortezomib monotherapy (median = 6.5 months)-hazard ratio = 0.55; 95% confidence interval = 0.43–0.71; P < .0001 (stratified log-rank test). The difference was considered clinically meaningful in this disease setting. The Kaplan-Meier TTP curves are shown in Figure 1. The response rate, including both complete and partial responses, was similar between the liposomal doxorubicin/bortezomib arm and the bortezomib monotherapy arm. However, the median duration of response was longer with combination therapy.
The overall survival analysis was not mature, as only 22% of subjects had died as of the data update at 4 months after the sNDA submission.
Safety
Safety was analyzed for the 636 patients who received at least one dose of the study drug. Treatment-emergent adverse reactions (TEARs) were generally comparable between the liposomal doxorubicin/bortezomib combination therapy and bortezomib monotherapy. As shown in Table 4, the most commonly observed adverse reactions, with a frequency of ≥ 25% on either arm, were fatigue, pyrexia, neutropenia, thrombocytopenia, anemia, nausea, vomiting, diarrhea, constipation, and peripheral neuropathy.
All-grade TEARs occurring with at least a 10% greater frequency in the combination arm compared to the bortezomib-alone arm were hand-foot syndrome, mucositis/stomatitis, and neutropenia. All-grade TEARS occurring with a 5% to 10% greater frequency in the combination therapy arm relative to the bortezomib-alone arm included fatigue, pyrexia, anorexia, thrombocytopenia, nausea, vomiting, diarrhea, weight decrease, and cough.
Grade 3/4 neutropenia occurred in 32% of subjects in the combination arm compared to 16% in the bortezomib arm, and thrombocytopenia occurred in 24% of subjects in the combination arm compared to 17% of subjects in the bortezomib arm. Grade 3 hand-foot syndrome was observed in 6% of subjects in the combination arm, but in no patients in the bortezomib-only arm.
The incidence of heart failure events was about 3% in both treatment arms. However, the protocol-defined decrease in LVEF was observed in 13% of subjects in the doxorubicin/bortezomib combination arm vs 8% in the bortezomib-alone arm.
The incidence of death within 30 days of treatment termination ascribed to study drug–related adverse reactions was 1% in both arms.
Discussion
The study supporting this liposomal doxorubicin supplemental application was an open-label, randomized, multicenter international trial. The interim analysis performed as prespecified in the study protocol demonstrated a statistically significant and clinically meaningful improvement in TTP with the liposomal doxorubicin/bortezomib combination as compared to bortezomib monotherapy. The response rates were similar between the two arms; however, the median duration of response was longer with the combination therapy compared with the monotherapy arm. The survival data were not mature. The safety analysis showed that TEARs associated with the combination appeared to be acceptable as compared to the monotherapy.
The review of this submission revealed that the data integrity was adequate and that the results of the sponsor's analyses were reliable. The baseline characteristics of patients and their disease characteristics were balanced between the two arms, especially for the factors known to influence the response to continued therapies and overall disease outcome. Since the determination of disease progression in most cases was based on centralized measurements of monoclonal proteins and a predefined algorithm, the possible TTP ascertainment bias associated with an open-label study was minimized. The participants in the trial were geographically heterogeneous and broadly representative of patients with progressive multiple myeloma, suggesting that the study results could be generalized to the patient population intended. On the other hand, major protocol violations and deviations occurred in about 10% to 15% of patients. However, several sensitivity analyses excluding those patients did not change the results of the ITT analysis of TTP.
The clinical significance of the LVEF decrease is not clear. The reversibility of the observed decreases in LVEF remained undefined, because the initial protocol design did not require follow-up of LVEF periodically after discontinuation of study medications. This should be taken into consideration when future trials are designed.
TTP has been used as an efficacy endpoint in assessing the effects of therapies for multiple myeloma.[3,9] Some evidence suggests that TTP correlates with survival in this disease setting,[10] especially when a large magnitude in TTP difference between treatment and control arms is observed. The regular approval of bortezomib was an example of employing TTP for regulatory action.[3] With the clinically meaningful difference in TTP observed in the current study, the combination of liposomal doxorubicin with bortezomib appears to confer a significant advantage in disease control. The observed median TTP of 6.5 months in the bortezomib arm is comparable to that (6.3 months) observed from the Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial that led to the approval of bortezomib for progressive multiple myeloma.[3,4] Although the overall survival data are not mature, the sponsor is committed to submitting updated survival data from DOXIL-MMY-3001 to the FDA once it is mature.
It is important to reiterate that the approval is for patients who have not received prior bortezomib, as no data were submitted supporting the effectiveness of the combination treatment with liposomal doxorubicin plus bortezomib in patients who had received bortezomib previously.
Conclusions
The evidence presented in this sNDA was substantial, included an adequate and well controlled study, and allowed assessment of both efficacy and safety of liposomal doxorubicin in combination with bortezomib in patients with progressive multiple myeloma who have received at least one prior therapy. The demonstrated benefits from the combination were felt to surpass the risks associated with its use in the intended patient population. The FDA review of the data and results corroborated the findings. Regular approval of this supplemental application was granted with the following indication: "Doxil in combination with bortezomib is indicated for the treatment of patients with multiple myeloma who have not previously received bortezomib and have received at least one prior therapy."
References:
1. Richardson PG, Hidesima T, Mitsiades, C, et al: The emerging role of novel therapies for the treatment of relapsed myeloma. J Natl Compr Canc Netw 5:149-162, 2007.
2. Kyle RA, Rajkumar SV: Multiple myeloma. N Engl J Med 351:1860-1873, 2004.
3. Kane RC, Farrell AT, Sridhara R, et al: United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 12:2955-2960, 2006.
4. Richardson PG, Sonneveld P, Schuster MW, et al: Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352:2487-2498, 2005.
5. Dimopoulos MA, Spencer A, Attal M, et al: Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): Results of a phase 3 study (MM-010) (abstract 6). Blood 106:6a, 2005.
6. Weber DM, Chen C, Niesvizky R, et al: Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): Results of a North American phase III study (MM-009) (abstract 7521). J Clin Oncol 24(18S):427s, 2006.
7. Bringhen S, Avonto I, Magarotto V, et al: Investigational treatments for multiple myeloma. Expert Opin Inverstig Drugs 15:1565-1582, 2006.
8. Updated DOXIL label. Available at www.fda.gov/cder/foi/label/2007/050718s029lbl.pdf. Accessed September 11, 2007.
9. Johnson JR, Williams G, Pazdur R: End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol 21:1404-1411, 2003.
10. Durie BGM, Jacobson J, Barlogie B, et al: Time to first progression, but not magnitude of regression predicts survival benefit in SWOG standard dose chemotherapy studies for multiple myeloma. J Clin Oncol 22:1857-1863, 2004.