The Three Most Common Chemotherapy-Related Skin Reactions
The increased approval of anticancer agents has led to unprecedented results, with improved quality of life and longer survival times, resulting in millions of individuals living with a diagnosis of cancer. Whereas these novel medical, surgical, and radiation regimens, or combinations thereof, are largely responsible for these remarkable achievements, a new, unexpected constellation of side effects has emerged. Most notably, cutaneous toxicities have gained considerable attention, due to their high frequency and visibility, the relative effectiveness of anti–skin toxicity interventions, and the otherwise decreasing incidence of systemic or hematopoietic adverse events. Optimal care dictates that dermatologic toxicities must be addressed in a timely and effective fashion, in order to minimize associated physical and psychosocial discomfort, and to ensure consistent antineoplastic therapy. Notwithstanding the critical importance of treatment-related toxicities, dermatologic conditions may also precede, coincide, or follow the diagnosis of cancer. This review provides a basis for the understanding of dermatologic events in the oncology setting, in order to promote attentive care to cutaneous health in cancer patients and survivors.
This article attempts to list the panoply of cutaneous manifestations or reactions that may be seen in cancer patients either as an epiphenomenon related to a cancer diagnosis or as a consequence of cancer treatment, particularly chemotherapy. The all-inclusive nature of the report would provide for a more effective presentation were it to focus on the three most common cutaneous reactions to chemotherapy: (1) hand-foot syndrome associated with the fluoropyrimidines; (2) taxane-associated acral erythema with onycholysis; and (3) the acneiform rash associated with the anti–epidermal growth factor receptor (EGFR) biologic agents. These three distinct and characteristic cutaneous reactions represent dose-limiting toxicities for these drugs and have a particular pathophysiologic mechanism that although largely unknown is probably not mediated by the immune system and has specific clinical characteristics, which are important from an oncologic perspective.
Hand-Foot Syndrome
The hand-foot syndrome was originally described in 1984 by Lokich and Moore.[1] The authors noted in a phase I study of protracted infusion of fluorouracil (5-FU) that 40% or more of patients developed a syndrome they referred to as palmar-plantar erythrodysesthesia. The authors did not initially recognize that the erythema of the palms and soles of patients was related to the continuous infusion of 5-FU, and patients were maintained on the infusion, resulting in a progressive evolution of the syndrome.
Therefore, the syndrome is not simply dose-related but is related to the duration of exposure over time. Thus, in patients receiving bolus (as opposed to protracted-infusion) 5-FU, hand-foot syndrome is rarely if ever recognized. The importance of duration of exposure is confirmed by the fact that a completely different antineoplastic agent-doxorubicin-if administered as a protracted infusion or delivered in the form of pegylated or liposomal doxorubicin (Doxil), can result in hand-foot syndrome. Although pegylated doxorubicin is administered as a bolus at 4-week intervals, the effect of pegylation is to protract the duration of exposure.
TABLE 1
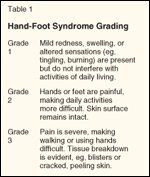
Hand-Foot Syndrome Grading
The hand-foot syndrome is associated with a grading system (see Table 1), reflecting the fact that increasing the duration of exposure increases the severity of the syndrome. Grade 1 hand-foot syndrome merely reflects some mild erythema and swelling on the palms with a minor sensation most probably secondary to subepidermal swelling. In the more severe grades of hand-foot syndrome, the palms and soles are affected with blister formation, profound discomfort, and the inability to hold objects or even to walk.
There is no known effective preventive measure for hand-foot syndrome, although one should never observe grade 3 or even grade 2 levels of severity since "real time" monitoring and detailed patient and family education about the syndrome and its early manifestations should prevent their appearance. Nonetheless, in the case of pegylated doxorubicin, the severity of the hand-foot syndrome may be more severe since there is no opportunity to stop the agent once it is administered. With infusion of 5-FU, the agent is cleared from the body with a half-life of 10 minutes precluding continued exposure, following treatment interruption.
Once the syndrome appears, topical therapies are the only treatments that may be useful. For example, Bag Balm (8-hydroxyquinoline sulfate 0.3% in a petrolatum and lanolin base), an emollient used by farmers for the hand rash that appears with milking udders, can be useful in the treatment of hand-foot syndrome.
Capecitabine (Xeloda) is a prodrug for 5-FU with an oral formulation and a drug schedule similar to the protracted parenteral infusion of 5-FU. The drug is administered twice daily for 14 days with a 7-day interval before the second cycle. Other schedules are being developed-for example, a 7-day-on, 7-day-off schedule-but the 14-day schedule continues to be associated with a 30% to 40% incidence of hand-foot syndrome.
The pathophysiologic mechanism for development of hand-foot syndrome has not been established. Histopathologic changes include vascular disruption and inflammation, but this is nonspecific. Furthermore, at a clinical level, the typical hand-foot syndrome as described above is seen with two distinctly different drugs: the antimetabolite 5-FU and the antibiotic doxorubicin. The common denominator is the protracted-infusion exposure to these two drugs.
Taxane-Associated Hand-Foot Syndrome or Acral Erythema
The cutaneous hand and foot reactions associated with the taxanes and particularly docetaxel (Taxotere) are often lumped together with the hand-foot syndrome associated with the fluoropyrimidines and doxorubicin. However, as Lokich and Childress reported in 2003,[2] the clinical manifestations on the extremities are quite different. The erythema appears on the dorsum of the hand as opposed to the volar (palm) surface and around the metacarpal joints. In addition, the thenar eminence is often involved with erythema as well as the periarticular area of the Achilles tendon. Finally, onycholysis is observed in more severe cases.
The taxane-associated hand-foot syndrome has been identified with the acronym PATEO (for periarticular thenar erythema with onycholysis). The mechanism or pathophysiology of PATEO syndrome is largely unknown. The severity of the syndrome is dose-related and has been mostly reported with docetaxel as opposed to paclitaxel. However, in a phase I study of the combination of docetaxel and paclitaxel, the frequency of PATEO was approximately 40%, reflecting the fact that the taxane moiety was the important element and not the distinctive diluents for each of the taxanes. The taxane diluents have been implicated as a cause of the hypersensitivity reactions associated with the taxanes.
The distinction between hand-foot syndrome associated with the taxanes and that associated with fluoropyrimidines perhaps is particularly important because a common regimen for the treatment of breast cancer is the combination of docetaxel and capecitabine, and the presentation of a hand-foot syndrome in a patient receiving these two agents could result in confusion and perhaps modification of the regimen of one of the drugs, depending upon the clinical pattern.
The hand-foot syndrome associated with the taxanes is related to dose, not schedule, and there is no preventive intervention. The only treatment is dose modification or treatment interruption or both.
Acneiform Rash Associated With Anti-EGFR Therapies
The anti-EGFR compounds and biologic agents or "targeted" therapies have recently been a major focus in oncologic therapeutics. There are two classes of EGFR inhibitors: the parenteral monoclonal antibody agents that function as a ligand for EGF receptors on the cell surface and the small-molecule tyrosine kinase (TK) inhibitors that affect the intracellular TK enzyme. The effect on the tumor cell is altered intracellular signal transduction and induced apoptosis along with other mechanisms that inhibit tumor growth.
The rash experienced by patients receiving one or another of these agents has the typical clinical appearance of acne with pustules and inflammation appearing on the face, scalp, and upper thorax. Histologically, however, the changes seen are not typical for acne, but are nonspecific. For all these agents, the effect observed is dose-related. It generally appears after two or more doses of the weekly or biweekly parenteral antibodies or 2 to 3 weeks after initiation of the small-molecule oral daily dosing agents.
There is no known preventive treatment that would preclude development of the acneform rash, and in fact, most patients receiving these agents develop a rash of varying severity. Dose modification or treatment interruption is effective in managing the problem, particularly for the oral agents. Real-time monitoring and anti-EGFR treatment interruption precludes the development of more severe manifestations of the rash. Treatment of the rash includes the use of antibiotics typically used for acne, and in spite of the different histologic appearance, this approach appears to be quite effective. Clindamycin lotion or gel are quite useful, and benzoyl peroxide (eg, Clearasil) has anecdotally been reported to improve the skin reaction in men.
An interesting aspect of the rashes associated with these agents is the correlation with tumor response. One retrospective study correlated tumor response with the severity of the rash, and a higher response rate was observed in patients with more severe grades of rash. Furthermore, one study increased the anti-EGFR dose to induce rash in patients with grade 0 or 1 rash and demonstrated that a dose increase leading to increased rash frequency and severity can result in increased likelihood of tumor response.
Conclusions
Oncologists are acutely aware of the above-cited categories of cutaneous reactions to chemotherapeutic intervention because of their high frequency and need to guide therapeutic dosing as well as timing based on the severity of the cutaneous effect. The therapeutic armamentarium for the oncologist in terms of the number of agents available is extensive. However, the frequency of cutaneous reactions to agents other than those described above is anecdotal and trivial in its consequences. Perhaps the most important preventive measure is for patients to be meticulously educated about the frequency and early recognition of treatment-associated rashes, to prevent severe manifestations of the dermatologic syndrome.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Lokich JJ, Moore C: Chemotherapy associated palmar-plantar erythrodysesthesia syndrome. Ann Intern Med 101:789-800, 1984.
2. Childress J, Lokich J: Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol 26:435-436, 2003.
3. Lokich J: Phase I clinical trial of weekly combined paclitaxel plus docetaxel in patients with solid tumors. Cancer 89:2309-2314, 2000.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.