Diffuse Large B-Cell Lymphoma: Changing Treatment Paradigms
With the progress in diagnostic methods that has made it possible to decipher the genetic code of DLBCL within a relatively short time, and with the increasing number of drugs that are entering clinical trials, our next big challenge is to enroll patients in trials in a timely manner.
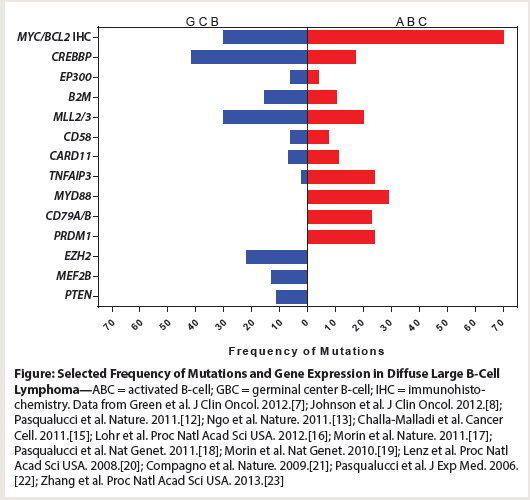
Figure: Selected Frequency of Mutations and Gene Expression in Diffuse Large B-Cell Lymphoma
Diffuse large B-cell lymphoma (DLBCL) was first cured with anthracycline-based chemotherapy in the 1970s. In the late 1990s, the addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy increased 10-year overall survival by approximately 15%. Two decades of variations on chemotherapy regimens and dosing schedules and upfront transplant have demonstrated no significant additional improvement. Despite the diversity inherent in DLBCL, the current paradigm of treatment is rituximab + CHOP (R-CHOP) for all patients with DLBCL.
While R-CHOP chemotherapy can cure approximately 50% of all patients with DLBCL, much work remains to be done to continue to improve these outcomes. Predictive models are needed to characterize the other 50% of patients who are not cured with the current standard regimens. There are two major types of predictive models in DLBCL. The first of these are the clinical models; the most widely used clinical model is the International Prognostic Index (IPI), which is based on age, tumor stage, serum lactate dehydrogenase concentration, performance status, and the number of extranodal disease sites. The second type are molecular models that utilize gene expression profiling.[1-3] The IPI predictive value is based solely on clinical and laboratory parameters and does not take into account the molecular heterogeneity of DLBCL.[3] The gene expression profiling models attempt to identify the molecular basis of DLBCL, using either a “cell of origin” model or a cellular stress model.[1,2]
Gene expression profiling has helped to further stratify patients beyond the IPI score, into molecular subtypes of DLBCL. The more commonly used cell of origin model classifies patients into germinal center B-cell (GCB) origin and activated B-cell (ABC) origin subtypes.[1] When treated with R-CHOP, patients with ABC DLBCL have worse outcomes and are candidates for novel treatment approaches. Accordingly, several clinical trials are selecting patients with ABC DLBCL for alternative therapy with lenalidomide, bortezomib, or ibrutinib added to the R-CHOP backbone.[4-6]
While this strategy has been successfully incorporated into clinical trials, grouping DLBCL patients into either ABC or GCB subgroups lacks mechanistic precision. ABC DLBCL can utilize several oncogenic pathways that are either redundant or cooperative; different treatments may be required depending on the oncogenic pathways involved. More recently, DLBCL that expresses high levels of MYC and BCL2 proteins (with or without associated chromosomal translocations) has been shown to have a poor prognosis when patients are treated with R-CHOP. Importantly, coexpression of MYC and BCL2 confers poor prognosis independent of the cell of origin.[7,8] This observation provided a new opportunity to explore new treatment strategies based on targeting MYC and BCL2.
Recent sequencing studies have identified recurrent genetic alterations that may also guide future therapy (Figure). Thus, it is now possible to subdivide DLBCL beyond the two groups based on cell of origin, into small subgroups that can be candidates for novel therapies. For example, patients with mutations in histone acetyltransferase genes (CREBBP and EP300) can be offered clinical trials with histone deacetylase (HDAC) inhibitors, those with EZH2 histone methyltransferase mutations can be offered clinical trials using EZH2 inhibitors, and patients with lymphoma cells that harbor CD79A/B mutations can be offered therapy with Bruton's tyrosine kinase (BTK) inhibitors or protein kinase C inhibitors.[9-13] While retrospective series show these mutations to be present in up to one-third of patients with DLBCL, prospective analysis will help verify the actual frequency of these mutations. For those patients with no identifiable mutations, the subtyping of DLBCL can be complemented by existing immunohistochemical methods. Immunohistochemistry (IHC) can identify DLBCL subtypes with constitutively active phosphoinositide 3-kinase (PI3K)/AKT pathways, phosphorylated mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways, and deregulated Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathways that do not harbor mutations. However, this will require standardization of the IHC methods across different clinical laboratories.
The subtyping of DLBCL, hindered in the past by the need for fresh frozen tissue for gene expression analysis, is made easier by the many technologies now available for molecular classification. In recent years, gene sequencing technologies have improved dramatically to allow for rapid sample turnover time and are able to sequence from paraffin-embedded tissue. As proof of principle, the NanoString platform was able to use formalin-fixed paraffin-embedded tissue for digital multiplexed gene expression to classify DLBCL within 24 hours, with no major discrepancies in the classification schema compared with traditional classification into GCB and ABC subtypes based on gene expression profiling.[14] These technologic advances will make it easier to develop user-friendly companion diagnostic tests that can be used to select patients for novel therapies.
With the progress in diagnostic methods that has made it possible to decipher the genetic code of DLBCL within a relatively short time, and with the increasing number of drugs that are entering clinical trials, our next big challenge is to enroll patients in trials in a timely manner. This will require active collaborations among different centers, not only to rapidly enroll patients, but also to openly share the new information gained from these trials.
Financial Disclosure:Dr. Younes receives research support from Curis, Johnson & Johnson, and Novartis; he receives honoraria from Bayer, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Sanofi, Seattle Genetics, and Takeda Millennium. Dr. Batlevi has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this commentary.
References:
1. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503-11.
2. Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851-61.
3. Project TIN-HsLPF. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987-94.
4. Nowakowski GS, LaPlant BR, Reeder C, et al. Combination of lenalidomide with R-CHOP (R2CHOP) is well tolerated and effective as initial therapy for aggressive B-cell lymphomas-a phase II study. 54th ASH Annual Meeting and Exposition; Dec 8-11, 2012; Atlanta, GA; abstr 689.
5. Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29:690-7.
6. Younes A, Flinn I, Berdeja J, et al. Combining ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); updated results from a phase 1b study in treatment-naïve patients with CD20-positive B-cell non-Hodgkin’s lymphoma (NHL). 55th ASH Annual Meeting and Exposition; Dec 7-10, 2013; New Orleans, LA; abstr 852.
7. Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460-7.
8. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452-59.
9. Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88-92.
10. McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108-12.
11. Naylor TL, Tang H, Ratsch BA, et al. Protein kinase C inhibitor sotrastaurin selectively inhibits the growth of CD79 mutant diffuse large B-cell lymphomas. Cancer Res. 2011;71:2643-53.
12. Pasqualucci L, Dominguez-Sola D, Chiarenza A, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189-95.
13. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115-9.
14. Masque-Soler N, Szczepanowski M, Kohler CW, et al. Molecular classification of mature aggressive B-cell lymphoma using digital multiplexed gene expression on formalin-fixed paraffin-embedded biopsy specimens. Blood. 2013;122:1985-6.
15. Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of beta2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20:728-40.
16. Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109:3879-84.
17. Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298-303.
18. Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830-7.
19. Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181-5.
20. Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105:13520-5.
21. Compagno M, Lim WK, Grunn A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717-21.
22. Pasqualucci L, Compagno M, Houldsworth J, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203:311-7.
23. Zhang J, Grubor V, Love CL, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2013;110:1398-403.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.