Large Renal Mass: A Challenge for the Urologist
Case History: 60-year-old man with mild right side abdominal discomfort and hepatomegaly found to have large right renal mass during CT scan.
Figure: Abdominal CT Scan With Oral and Venous Contrast
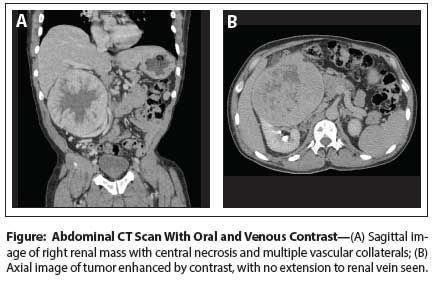
The Case: A 60-year-old man was incidentally found to have a large right renal mass during a CT scan with and without intravenous and oral contrast ordered by his primary care physician to evaluate mild right side abdominal discomfort and hepatomegaly. Medical and surgical history was significant for scoliosis with chronic lower back pain, as well as gastric cancer treated with surgery and chemotherapy more than 15 years ago. The patient’s family history included diabetes mellitus type 2 and breast cancer in his mother, but no history of renal or prostate cancer.
A CT scan (Figure) showing the right kidney contained a very large, 16.23 × 11.9-cm anterior-inferior enhancing renal mass, with a large central area of necrosis, as well as what appeared to be a renal cell carcinoma (RCC) with multiple small lytic lesions through the lumbar spine and pelvis, suspected to be osseous metastases. A few scattered calcifications within the tumor were noted. The mass was surrounded by multiple vascular collaterals. There was no extension of the tumor into the renal vein. A CT scan of the thorax revealed a 2-mm upper-lobe nonspecific pulmonary nodule and a 4-mm lytic abnormality in the right anterior T2 vertebral body. An MRI did not reveal the presence of a renal vein thrombus but reported enlarged venous collaterals around the margins of Gerota’s fascia, extending to the right iliac vein. Lymphadenopathy was present. Laboratory results, including the alkaline phosphatase level, were in the normal range.
The patient underwent laparoscopic radical nephrectomy after options had been discussed. A conventional transperitoneal laparoscopic radical nephrectomy was performed. During the procedure, enlarged lymph nodes were observed in the paracaval and major renal vessels regions, and extensive bowel adhesions from a previous surgery were also seen. Total blood loss was 400 mL, and the procedure was performed with no evident complication.
The patient’s pathology examination revealed a 16.2-cm oncocytoma with a low nuclear grade in the upper and mid portion of the kidney, without sarcomatoid features, focally involving a branch of the renal vein. Perirenal fat and surgical margins were negative. Papillary adenomas were found in the renal cortex, the largest being 8 mm. Hilar and retroperitoneal lymph nodes were negative, as was the right adrenal gland. Tumor cells were negative for vimentin and CK7, and were also negative on colloidal iron staining. Immunostaining for CD10 showed focal staining and was noncontributory.
According to American Joint Committee on Cancer staging criteria, the tumor corresponded to TNM pathologic stage pT3a N0 MX. No sarcomatoid features were noted. No other tumor components were identified.
The patient was discharged to home on postoperative day 5, after an ileus resolved spontaneously. A CT bone windows study showed that the bone lesion was related to degenerative disease.
Discussion
In general, large solid renal masses that are enhanced with intravenous administration of contrast agents on CT by more than 15 Hounsfield units (HU) should be regarded as RCC until proven otherwise.[1]
An oncocytoma is the most common benign tumor that appears as an enhancing renal mass on cross-sectional imaging; it is extremely challenging to diagnose preoperatively, and thus must be presumed to be RCC until surgical excision.[2] Recently, genotyping studies confirmed oncocytoma to be a benign histology with a distinct cell of origin and genetic abnormalities.[3] Cases of metastatic disease have been reported, but are considered exceptionally rare and may represent cases of malignant degeneration or pseudometastases.[4,5]
It has been reported that oncocytomas may demonstrate malignant behavior, but only if associated with cytonuclear and histologic features indicative of malignancy, which were not seen in this case.[5,6]
Genetically, oncocytomas present with a pattern of alterations in chromosome 1, with heterozygosity for chromosomes 1 and/or X and/or 14, similar to the pattern seen in chromophobe RCCs, which also carry additional chromosomal changes.[7] In the case of oncocytosis, a hybrid oncocytoma/chromophobe RCC is identified. With genetic support, oncocytomas can progress to chromophobe RCC.[7]
Since 1985, when Bosniak proposed a classification of cysts and cystic renal masses in an attempt to differentiate patients who need surgical treatment and follow-up from those who do not, accurate diagnosis of renal lesions has been achieved with CT-and with MRI in patients with indeterminate lesions on CT.[8,9]
Clayman et al described the first laparoscopic radical nephrectomy to treat oncocytomas, and since then this procedure has quickly become one of the most common procedures used to treat renal masses.[10] Experience with laparoscopic equipment makes it possible for a surgeon to use minimally invasive procedures to treat large renal masses, with better perioperative outcomes and without compromise of oncologic outcomes.[11]
Oncocytomas represent a challenge for the urologist, from the diagnosis through treatment and follow-up. A better understanding of this disease is needed to fill the current knowledge gap and thus to improve treatment and outcomes.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Hartman DS, Choyke PL, Hartman MS. From the RSNA refresher courses: a practical approach to the cystic renal mass. Radiographics. 2004;24(suppl 1):S101-15.
2. Morra MN, Das S. Renal oncocytoma: a review of histogenesis, histopathology, diagnosis and treatment. J Urol. 1993;150:295-302.
3. Kuroda N, Toi M, Hiroi M, et al. Review of renal oncocytoma with focus on clinical and pathobiological aspects. Histol Histopathol. 2003;18:935-42.
4. Paner GP, Turk TM, Clark JI, et al. Passive seeding in metanephric adenoma: a review of pseudometastatic lesions in perinephric lymph nodes. Arch Pathol Lab Med. 2005;129:1317-21.
5. Oxley JD, Sullivan J, Mitchelmore A, Gillatt DA. Metastatic renal oncocytoma. J Clin Pathol. 2007;60:720-2.
6. Perez-Ordoñez B, Hamed G, Campbell S, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol. 1997;21:871-83.
7. Van der Kwast T, Perez-Ordoñez B. Renal oncocytoma, yet another tumour that does not fit in the dualistic benign/malignant paradigm? J Clin Pathol. 2007;60:585-6.
8. Hartman DS, Aronson S, Frazer H. Current status of imaging indeterminate renal masses. Radiol Clin North Am. 1991;29:475-96.
9. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
10. Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy. N Engl J Med. 1991;324:1370-1.
11. Berger A, Brandina R, Atalla MA, et al. Laparoscopic radical nephrectomy for renal cell carcinoma: oncological outcomes at 10 years or more. J Urol. 2009;182:2172-6.