DIRI Promising in Evaluating Drug Response
SAN FRANCISCO-A highly sensitive photon sensor has shown promise as a means of detecting early, subtle responses to neoadjuvant therapy among patients with soft tissue sarcomas, investigators from the Dana-Farber Cancer Institute reported at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO).
SAN FRANCISCOA highly sensitive photon sensor has shown promise as a means of detecting early, subtle responses to neoadjuvant therapy among patients with soft tissue sarcomas, investigators from the Dana-Farber Cancer Institute reported at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO).
Technology Transfer: From NASA to Medical Centers
The photon sensor used in OmniCorder’s BioScan System for dynamic infrared imaging was originally developed by NASA’s Jet Propulsion Laboratory (JPL) and the California Institute of Technology (Pasadena, California) for the never-completed Strategic Defense Initiative (SDI) program, also known as "Star Wars."
The sensor, called the Quantum Well Infrared Photodetector (QUIP) was invented by Dr. Sarath Gunapala, principal engineer of JPL’s Device Research and Applications Section. OmniCorder obtained exclusive licensing rights for medical applications of the sensor in 1998 as part of the federal government’s technology transfer program.
The new modality, known as dynamic infrared imaging (or DIRI), is based on infrared-detection technology developed for the "Star Wars" missile defense system (see box).
Complex Morphology
Sarcomas often present with complex morphology that is difficult to evaluate on CT or MRI, said presenter Milos Janicek, MD, PhD. Dynamic infrared imaging, he said, "is a very sensitive, rapid, and precise method" of monitoring infrared emission data from the tumor site or adjacent soft tissues that can show changes in blood perfusion. He noted that the prognostic significance of metabolic and perfusion changes in sarcomas "is very intriguing."
The Dana-Farber researchers are using the BioScan System (OmniCorder Technologies, Stony Brook, NY), which consists of a digital infrared camera that "counts" photons (electromagnetic radiation) emitted from tissues and organs, and sophisticated computer software that analyzes changes in the photon flux to map patterns of blood perfusion in tissues (see Figure 1). The results can be viewed on a monitor or printed out as hard copy.

The device has been cleared for marketing by the FDA for use as adjunct diagnostic screening for detection of breast cancer and diseases affecting the blood perfusion or reperfusion of tissue or organs.
How It Works
Cancer cells produce a variety of vasoactive substances such as nitrous oxide, which lead to increased blood flow in tissues surrounding the cancer. The sensor detects the infrared energy (photons) emitted from the body, thus "seeing" the minute changes in tissue blood perfusion or reperfusion caused by cancer metabolic activity.
Production of vasoactive substances and tissue mediators begins earlier than measurable mass, which is detected by x-ray or CT.
The BioScan System is sensitive to changes in infrared photon flux that are equivalent to temperature changes of less than .009 of a degree centigrade and has a speed of more than 200 frames per second. A typical digital infrared exam takes about 20 seconds.
George Demetri, MD, medical director of the Center for Sarcoma and Bone Oncology at Dana-Farber, and one of the study authors, said that infrared imaging may be particularly useful in measuring responses to new antiangiogenesis agents under study. Because of its safety, speed, and low cost, dynamic infrared imaging can be performed frequently in studies of new drugs.
Dr. Janicek suggested that antiangiogenic effects are not specific to so-called antiangiogenic agents, but, rather, may be common to large groups of therapies that may have a toxic effect on the newly formed blood vessels. "Here, digital infrared imaging might make a powerful contribution due to its ability to monitor perfusion-related changes linked to tissue physiology and cell growth or death," he said.
Dr. Demetri stressed that the technique documents blood vessel oscillations, as opposed to simply heat. "Our normal blood vessels are oscillating up and down in a certain way, and it turns out that tumor blood vessels go through that rhythm in a very confused and very different way, registering a very different pattern that can be seen with digital infrared imaging," Dr. Demetri said. "It has nothing to do with temperature. The temperature of two parts of the body might be exactly the same, but the pattern of oscillation is very different."
The Dana-Farber researchers performed 27 sets of CT scans, FDG-glucose PET scans, and digital infrared images (at baseline and at 1-month intervals) in 14 patients with soft tissue sarcomas receiving a variety of drug treatments. On digital infrared imaging, tumor masses were identified as areas of increased heat distribution and increased modulation (or oscillation) of temperature (blood vessel oscillations).
Sarcomas were selected for the study, many of them large but often relatively superficial. It was assumed that lesions relatively close to the surface of the body would be more likely to register changes on infrared imaging.
Visual examination of digital infrared images correctly suggested clinical progression or regression of tumors after treatment in 75% of lesions, Dr. Janicek said. "For those tumors that responded to treatment, there was decreasing temperature modulation between baseline and rescanning 1 month later, whereas progressive tumors showed statistically significant increases in modulation," he said (see Figure 2).
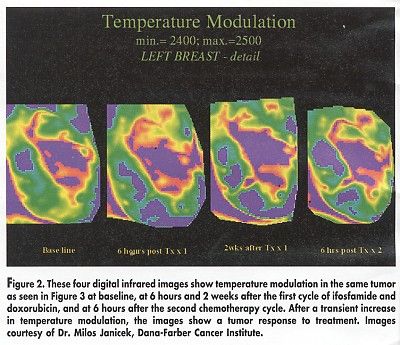
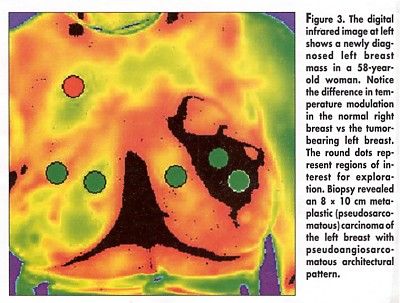
Dr. Janicek noted that baseline temperature did not predict treatment response. "All tumors show similar warmer temperature, compared with background," he said. Temperature modulation, however, may have prognostic potential, since baseline modulation was greater for those tumors that subsequently responded to treatment, he said (see Figure 3).
Long Follow-up Planned
He concluded that infrared imaging could add information on the biology of the tumor. "It monitors tumors with a noninvasive, easy-to-reproduce approach, provides both quantitative and qualitative assessment, and may even have predictive value at baseline, allowing for selection of patients," Dr. Janicek said.
He noted that patients will be followed for several years in order to correlate the prognostic information from the infrared images with clinical outcomes.
The discussant for the sarcoma session, Douglas L. Fraker, MD, University of Pennsylvania, called the new technique "an important way to look at tumors."
Instead of giving neoadjuvant therapy and seeing if patients respond, Dr. Fraker said, physicians might be able to predict a response by looking at the temperature modulation in the tumor before treatment. Another method to accomplish this may be by looking at tumor hypoxia, he commented.
In an interview, Mark Fauci, MBA, president and CEO of OmniCorder, said that the BioScan System is being tested in a variety of other settings, including use during brain surgery to "see" blood perfusion changes around the margins of brain lesions and in response to stimulus of different areas of the brain.
Overcoming ‘Brain Shift’
At the Mayo Clinic, Rochester, Minnesota, he said, surgeons are investigating digital infrared imaging as a means of overcoming the problem of "brain shift" during lengthy surgeries. Once the skull is opened, the brain and thus the location of the tumor may shift by centimeters, rendering the preoperative MRI data on tumor location imprecise, he said.
"The bottom line," Mr. Fauci said, "is that cancer therapy monitoring is only one of many applications that we’re working on."