Subcutaneous Amifostine Provides Protection Against Radiation-Induced Acute Xerostomia
PHILADELPHIA-For head and neck cancer patients, subcutaneous (SC) amifostine (Ethyol) provides equal protection against radiation-induced grade 2 acute xerostomia compared to intravenous (IV) amifostine, Pramila Rani Anné, MD, reported. She cautioned, however, that SC amifostine should be used only in clinical trials until ways to prevent cutaneous toxicities are worked out.
PHILADELPHIAFor head and neck cancer patients, subcutaneous (SC) amifostine (Ethyol) provides equal protection against radiation-induced grade 2 acute xerostomia compared to intravenous (IV) amifostine, Pramila Rani Anné, MD, reported. She cautioned, however, that SC amifostine should be used only in clinical trials until ways to prevent cutaneous toxicities are worked out.
Dr. Rani Anné, instructor in the Department of Radiation Oncology at Thomas Jefferson University Hospital in Philadelphia, presented phase II data comparing SC and IV amifostine in patients being treated with radiation therapy for head and neck cancer. The study followed trial WR-38, which showed that IV amifostine significantly reduced moderate-to-severe radiation-induced xerostomia in patients with head and neck cancer.
The SC formulation attracted attention after pharmacokinetic studies (WR-57) showed 50% dose-normalized bioavailability compared to IV dosing, with no reported nausea/vomiting or hypotension. The phase I comparison of IV, oral, and SC amifostine in a 3-way crossover study in 12 healthy volunteers showed similar plasma concentrations of WR-1065, the active metabolite of amifostine, apart from an early peak seen with the IV but not with the other regimens.
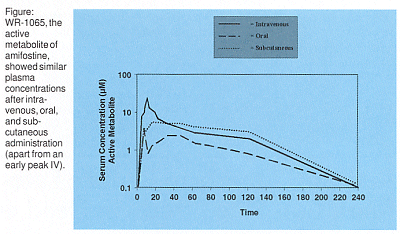
What’s Behind the Peak?
"This peak is thought to relate to hypotension and nausea. The question is whether it is also important for xerostomia protection," Dr. Rani Anné said.
Dr. Rani Anné’s phase II study with SC amifostine (WR-60) was undertaken in part to answer that question. The trial was designed to permit comparison with the previous study of IV amifostine (WR-38). "The primary study objective was to look at the effect of SC amifostine on incidence of grade 2 or worse acute xerostomia, assessed using the Radiation Therapy Oncology Group (RTOG) criteria," she said. Secondary objectives were effects on the incidence of grade 3 or worse acute mucositis, grade 2 or worse late xerostomia, and toxicity.
WR-60 enrolled 55 patients with newly diagnosed squamous cell head and neck cancer scheduled for radiotherapy to at least 40 Gy that would include at least 75% of both parotid glands. Patients could have no evidence of distant metastases and could not have had prophylactic pilocarpine.
Evaluable Patients
Dr. Rani Anné reported data on 54 patients who had been treated and were evaluable for acute toxicity. Patient characteristics were quite similar to those of patients in the WR-38 IV amifostine study used for comparison.
"Patients all received amifostine 500 mg SC in 2.5 mL of solution, which was given by two injections in two locations, at 60 minutes plus or minus 15 minutes prior to radiation," Dr. Rani Anné said. "Radiation was 1.8 to 2 Gy per day times 5 days, to a total of 50 to 70 Gy."
The median duration of radiotherapy was 45 days. Median number of fractions was 32, and the median dose was 62 Gy. Comparable data from the WR-38 IV amifostine study were: duration of radiotherapy 48 days, median of 32 fractions, and median total dose of 64 Gy.
Dr. Rani Anné said that 57% of patients had grade 2 acute mucositis and 33% had grade 3 acute mucositis after SC amifostine. "This was expected and appears fairly similar to what would be anticipated if patients did not receive IV amifostine. By radiation dose, patients who received over 60 Gy had a higher incidence of grade 3 mucositis," she said.
There was no grade 3 nausea with SC amifostine, compared to the 3% in the IV amifostine study, but there was a significant amount of mild nausea (81% vs 44% with IV amifostine). Vomiting was similar with SC and IV amifostine. There was no grade 3 hypotension with SC amifostine vs 3% with IV amifostine.
Injection Site Reaction
"The biggest problem with SC amifostine was injection site reaction and rash. There was a 29% incidence of injection site reaction, but it did not bother most of the patients. It was typically either mild redness or slight erythema or pain around the injection site. With IV amifostine the incidence of this rash was only 1%. With SC amifostine the incidence of grade 3 rash was 13%," Dr. Rani Anné said.
"Twenty-four percent of SC amifostine patients had a grade 1 rash vs 4% in the IV amifostine study," she continued. "Sixteen of the 54 patients who took SC amifostine had an injection site reaction. None of these were grade 3 or ulcerative systemic reactions. However, six patients (11%) had a maculopapular rash, four (7%) of which were over the entire body and classed as grade 3. Two patients had grade 3 urticarial rash, and one patient had grade 3 erythema multiforme."
The reductions in grade 2 acute xerostomia with SC amifostine appear comparable to those achieved with IV amifostine in head and neck cancer patients treated with radiotherapy. The SC treatment was generally well tolerated, with less severe nausea, vomiting and hypotension than with IV amifostine. "Cutaneous toxicity was more frequent with SC amifostine than with IV administration, and until we develop therapeutic interventions for the systemic rash, routine use should be limited to protocol patients," Dr. Rani Anné said.