Effectiveness, Toxicity, and Survival Predictors of Regorafenib in Metastatic Colorectal Cancer: A Multicenter Study of Routinely Collected Data
María Pérez Abánades, PharmG
- Silvia Ruíz-García, PharmG
- Ana Beatriz Fernández Román, PharmG
- Javier Letellez Fernández, PharmG
- Beatriz Candel García, PharmG
- Carlos Hernández Terciado, PharmG
- Raquel De Santiago Álvarez, PharmG
- Rebeca Mondéjar Solis, MD
- Berta Hernández Marin, MD
- Patricia Toquero Diez, MD
- Alberto Morell Baladrón, PharmG
- Ramón Colomer Bosch, MD
Alberto Calvo-García, PharmG, and colleagues analyzed routinely-collected data to assess regorafenib in metastatic colorectal cancer.
ABSTRACT
OBJECTIVES: To assess the effectiveness and toxicity of regorafenib in patients with metastatic colorectal cancer (mCRC) in routine clinical practice, as well as predictive factors of effectiveness.
METHODS: This was a retrospective multicenter study in patients with mCRC who received regorafenib from November 2013 to May 2020. Effectiveness was evaluated by overall survival (OS) and progression-free survival (PFS) using the Kaplan-Meier method. Cox regression was performed to determine survival predictors.
RESULTS: Ninety patients were enrolled (median age, 64.3 years). Fifty-two patients (57.8%) were male, and 57 (63.3%) had an ECOG performance status (PS) of 0 to 1. Median follow-up was 2.80 months. Median OS was 8.03 months (95% CI, 5.90-10.17), and median PFS was 2.90 months (95% CI, 2.59-3.21). Eighty-eight patients (97.8%) experienced drug-related adverse events. The most frequent were fatigue in 66 patients (73.3%), followed by palmar-plantar erythrodysesthesia in 40 (44.4%). Low liver tumor burden score (LTBS) and good ECOG PS were independent OS predictive factors.
CONCLUSIONS: Patients taking regorafenib had OS and PFS rates similar to those reported in previous randomized trials; the agent had a poor toxicity profile. We identified low LTBS and good ECOG PS as possible predictive factors of better OS, useful in selecting patients with mCRC who might benefit from regorafenib.
Oncology (Williston Park). 2022;36(12):732-738.
DOI: 10.46883/2022.25920981
Introduction
Colorectal cancer (CRC) is a heterogeneous disease that involves a wide variety of driving mutations.1 According to GLOBOCAN, CRC was the third most common cancer and the second most common cause of cancer death worldwide in 2020.2 In United States (US) and Spain, approximately 155,098 and 37,172 new cases of CRC were diagnosed in 2018, respectively.3,4
The current treatment of choice for metastatic CRC (mCRC) is chemotherapy combined with antibodies against either VEGF or EGFR.5 Despite the treatment options, CRC mortality represented the 8.6% and 9.2% of all deaths caused by cancer in 2018 in US and Spain, respectively.3,4
Regorafenib is an oral multikinase drug currently approved for use in third-line therapy of mCRC. It inhibits tyrosine kinase receptors involved in tumor angiogenesis (VEGFR1/3 and TIE-2), oncogenesis (c-KIT, RET, RAF-1, BRAF, and BRAF V600E), metastasis (PDGFR-b, FGFR-1, and VEGFR3) and immunity (CSF1R).6 The dose approved by the Food and Drug Administration is 160 mg per day for 21 days in 28-day cycles.7
Regorafenib has demonstrated an increase in overall survival (OS) compared with placebo in two phase 3 clinical trials in patients with mCRC who had previously become refractory to fluoropyrimidine-based chemotherapy, anti-VEGF therapy, and anti-EGFR therapy. The median OS with regorafenib was 6.4 months (vs 5.0 with placebo) in the CORRECT trial (NCT01103323) and 8.8 months (vs 6.3 with placebo) in the CONCUR trial (NCT01584830). The median progression-free survival (PFS) was 1.9 months (vs 1.7 months) and 3.2 months (vs 1.7 months), respectively.8,9 In both trials, drug-related adverse events (AEs) of grade 3 or higher occurred in 54.0% of patients. Palmar-plantar erythrodysesthesia was the most frequent AE, occurring in 17.0% and 16.0%, respectively.8,9
On the European Society for Medical Oncology (ESMO)-Magnitude of Clinical Benefit Scale (MCBS), which measures the clinically significant benefit that can be expected from cancer treatments, regorafenib as third-line treatment of mCRC received a score of 1, indicating low clinical benefit.10
The primary outcome of the present study was to evaluate the routine clinical practice use of regorafenib, evaluating its effectiveness and safety. As a secondary objective, we aimed to establish predictive factors for survival.
Methods
Study Design
A multicenter retrospective study was conducted in 3 Spanish hospitals (La Princesa University Hospital, Fuenlabrada University Hospital, and Puerta de Hierro University Hospital). The inclusion criteria were being 18 years or older, diagnosed with mCRC, and beginning treatment with regorafenib between November 2013 and May 2020. Patients participating in any clinical trial were excluded. Data were collected in September 2020 to have a minimum treatment period per patient of 4 months.
Variables
Sociodemographic, clinical, hematological, and biochemical variables from patients were collected from the electronic medical records. The main efficacy variables were OS (defined as the length of time from the initial administration of regorafenib until the last control performed before the study ended or patient death) and PFS (defined as the time between the beginning of treatment and clinical or radiological disease progression). Progression was evaluated by CT scan every 3 months. To evaluate the safety of the drug, AEs were recorded, including those reflected in the clinical history and test results as well as those reported by the patients in pharmaceutical care and oncology consultations. The blood parameter alterations were defined according to the normal limit values and the specifications of the drug.7 Drug-related AEs were graded using the Common Terminology Criteria for Adverse Events, version 5.0.11
To identify potential predictive response factors to regorafenib in relation to OS, we evaluated clinical parameters, the patient’s functional status measured by ECOG performance status (PS),12 and the liver tumor burden score (LTBS), which is calculated by the number of liver metastases and the diameter of the largest lesion obtained from radiologists’ report data.13 Patients were divided into 3 groups: those with LTBS lower than 3 (LTBS-low; low liver tumor burden), LTBS 3 to 9 (LTBS-med; medium liver tumor burden), and LTBS higher than 9 (LTBS-high; high liver tumor burden).13 Patients in whom LTBS was not available were classified as unknown.
Statistical Analysis
Normal distribution variables were calculated as proportions, means, and SD; nonnormal distribution variables were calculated as medians and IQRs. A Kaplan-Meier survival analysis was carried out to evaluate OS and PFS. To establish how clinical and pathological variables influenced OS and PFS, univariate and multivariate Cox regressions, keeping OS and PFS as independent variables, were performed. Only variables with P less than or equal to .05 for the OS were included in multivariate analysis, with the exception of the liver metastasis variable, which was not included because it was a dependent variable of the LTBS variable. The results were considered statistically significant if P less than or equal to .05. The software used was SPSS version 22.0.
Results
Sociodemographic and Clinical Characteristics
The study included 90 patients treated with regorafenib; 52 (57.8%) were male, with a mean age of 64.3 (SD, 9.3) years. Table 1 shows sociodemographic characteristics and Table 2 shows clinical baseline characteristics.
TABLE 1. Sociodemographic Characteristics
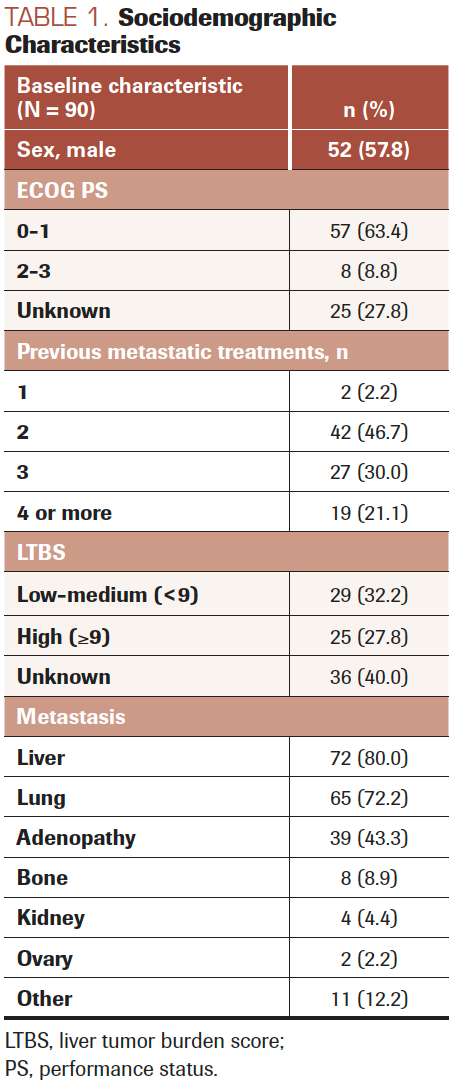
The median number of pretreatment lines was 2.9 (SD, 1.2). The median time of treatment was 2.8 (range, 1.0-2.8) months. Fifty-five patients (61.1%) started with the full initial dose of 160 mg, 18 (20.0%) with 120 mg, 16 (17.8%) with 80 mg, and 1 patient (1.1%) with 40 mg of regorafenib. Thirty-one patients (34.4%) did not receive full doses of regorafenib at any time during treatment.
TABLE 2. Clinical Baseline Characteristics
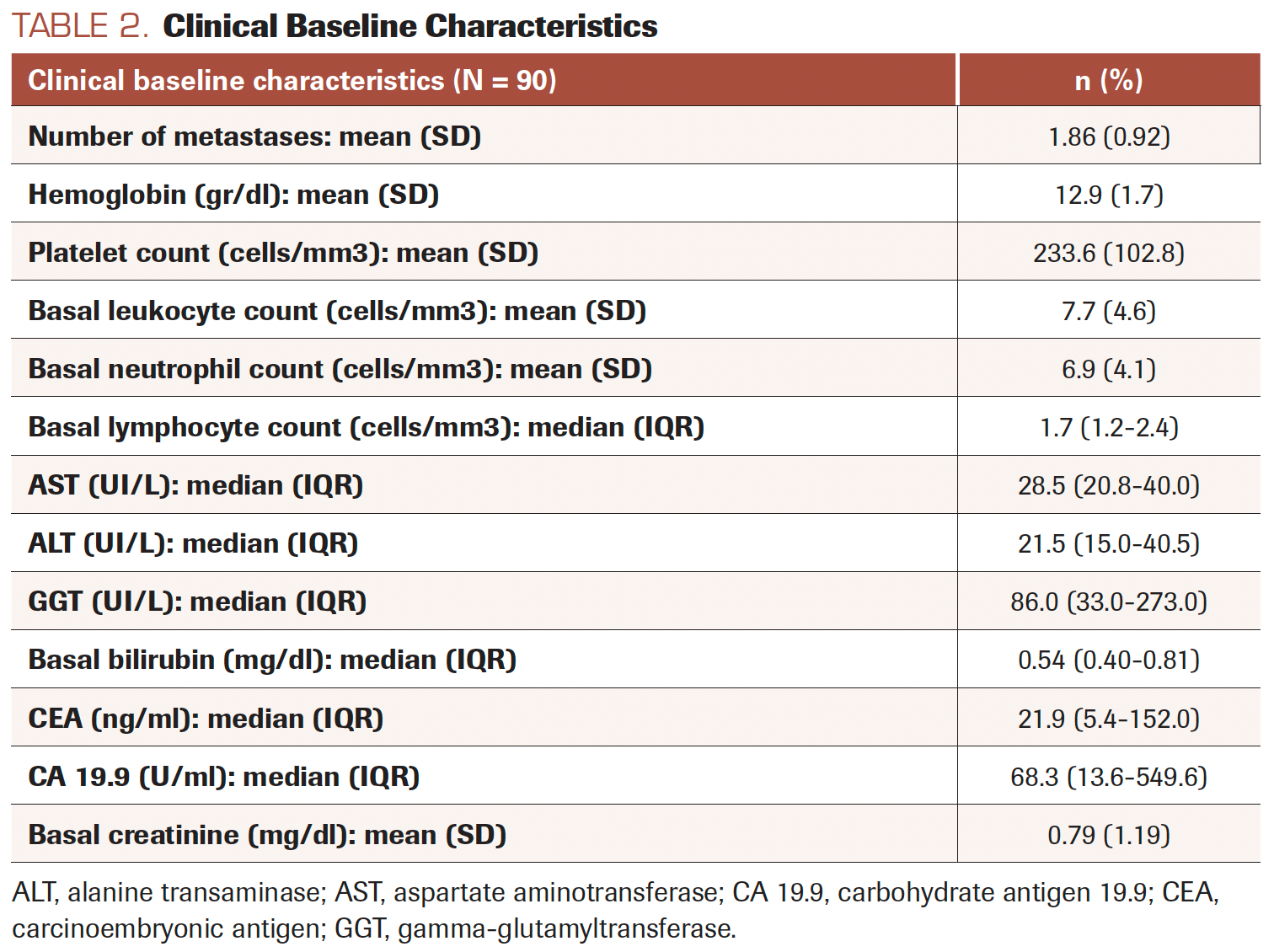
FIGURE 1. Progression-Free Survival (PFS) and Overall Survival (OS) Kaplan-Meier Curves of Regorafenib
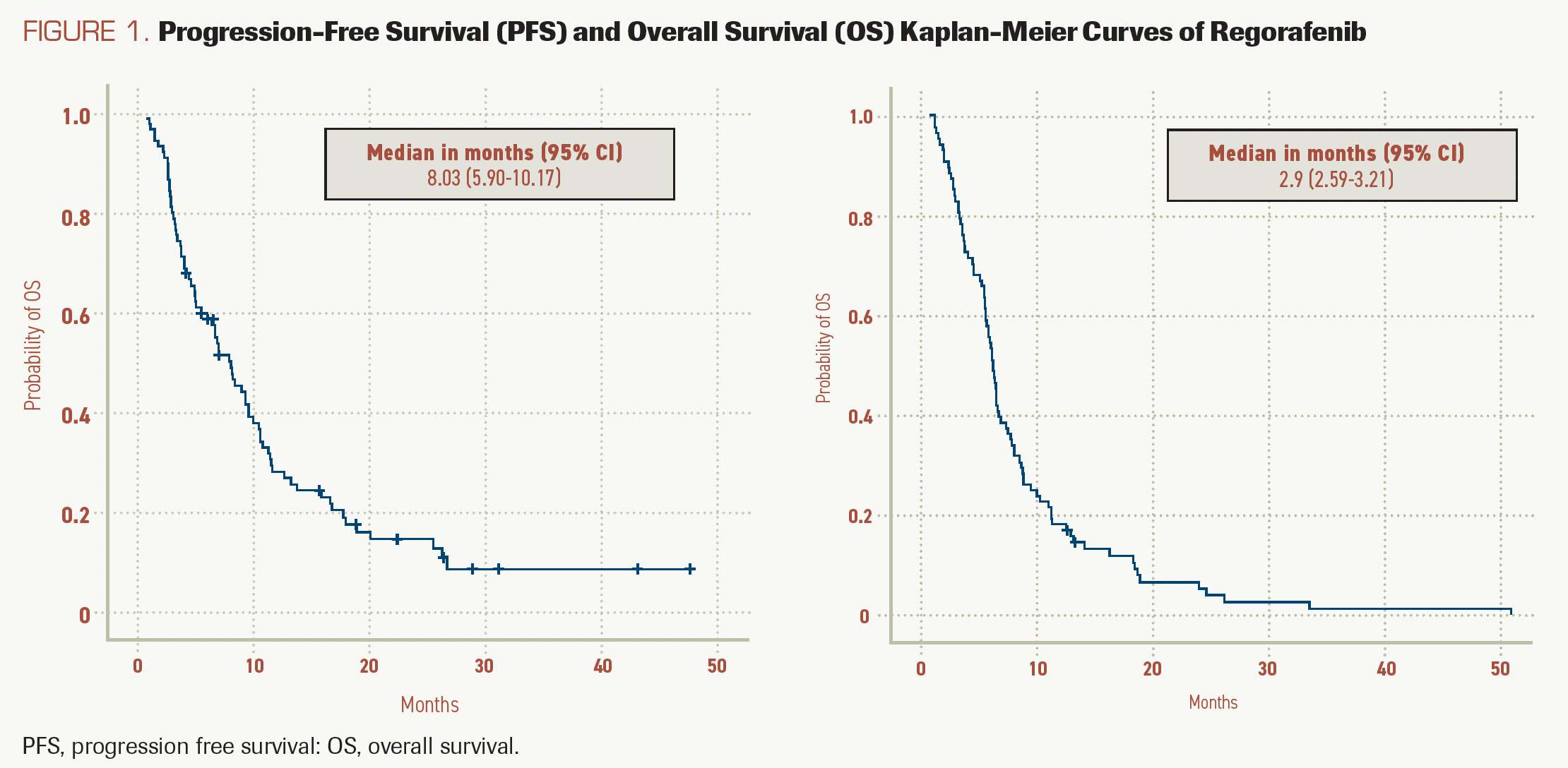
FIGURE 2A.
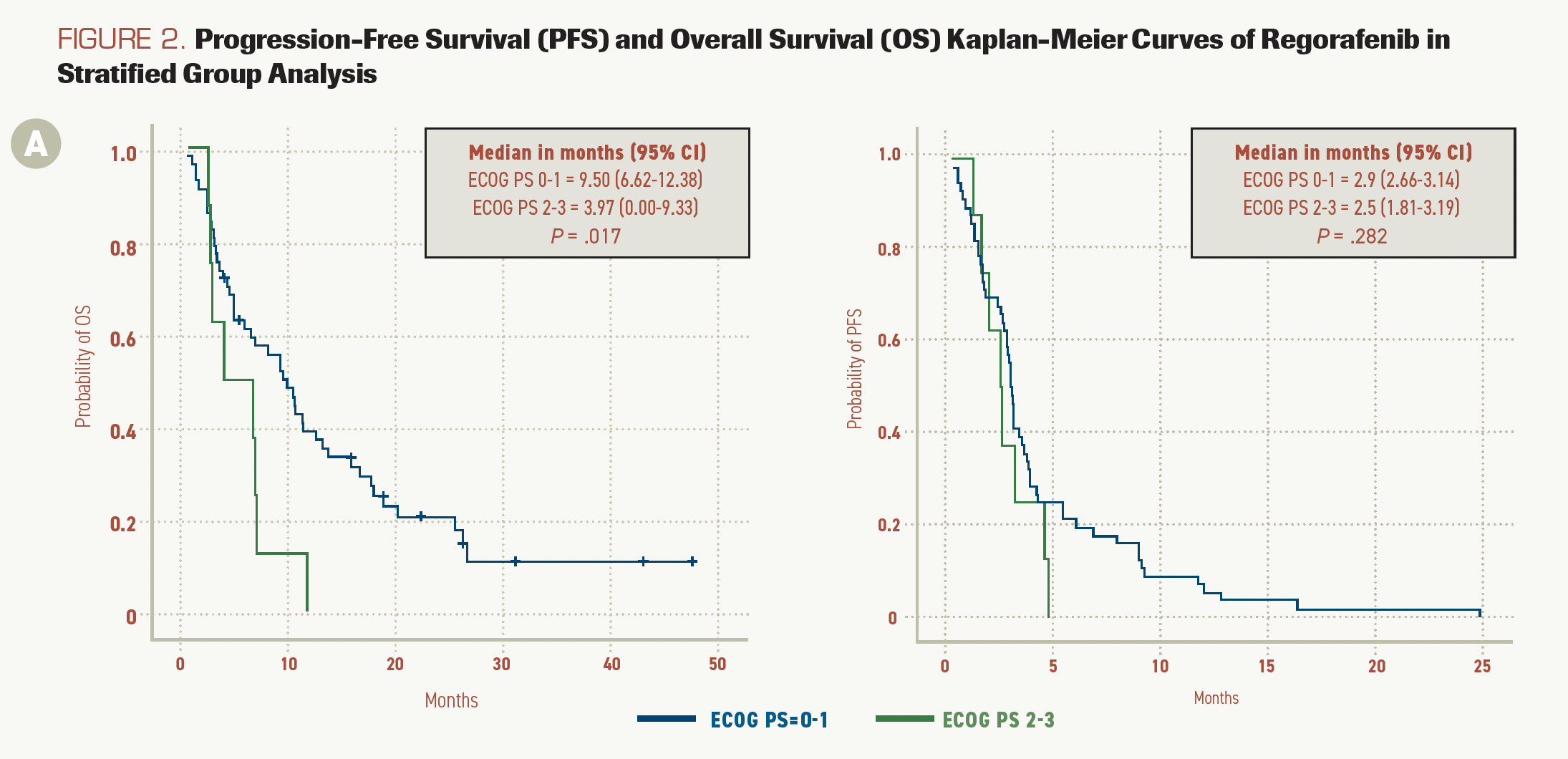
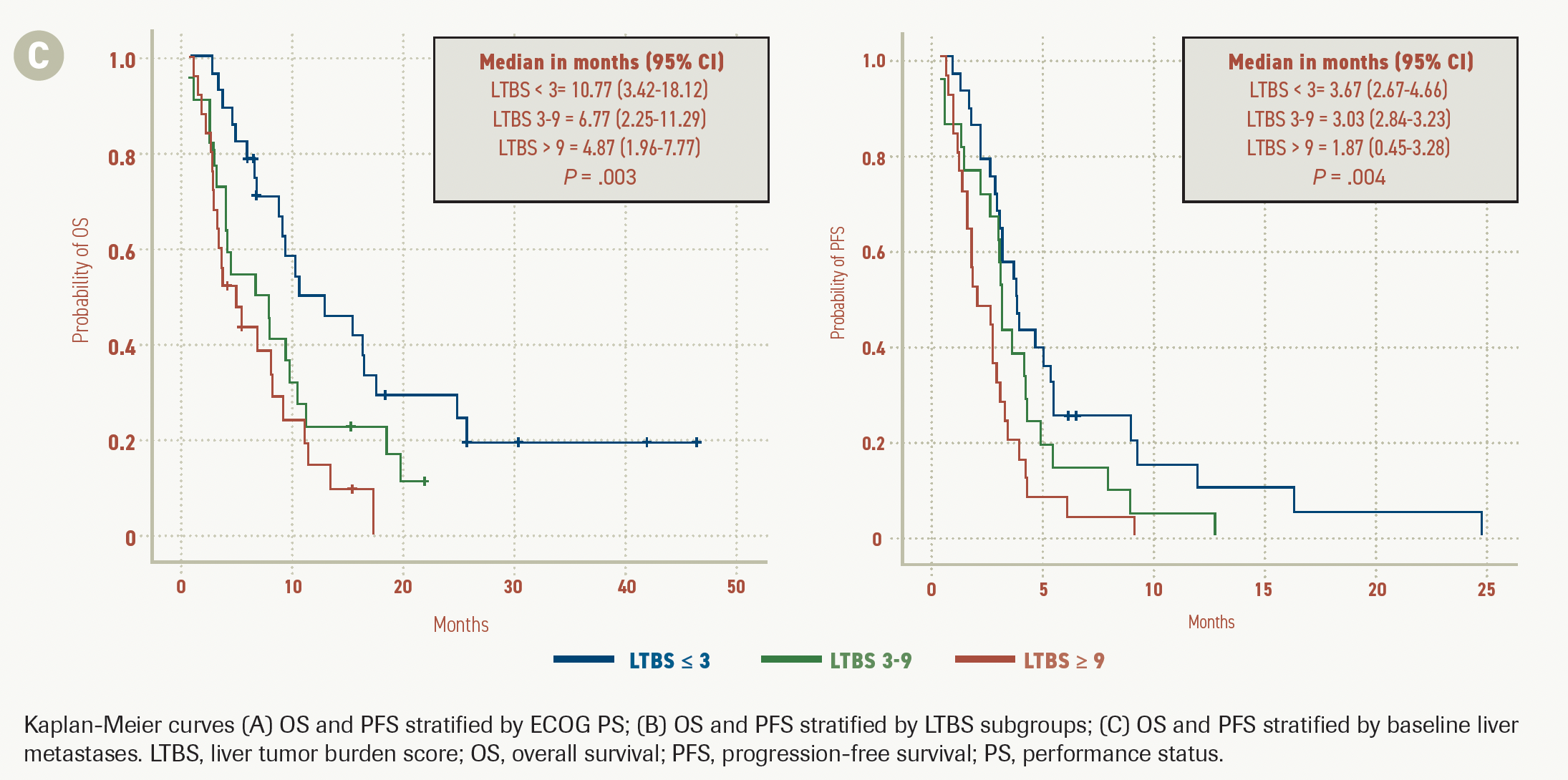
FIGURE 2B.
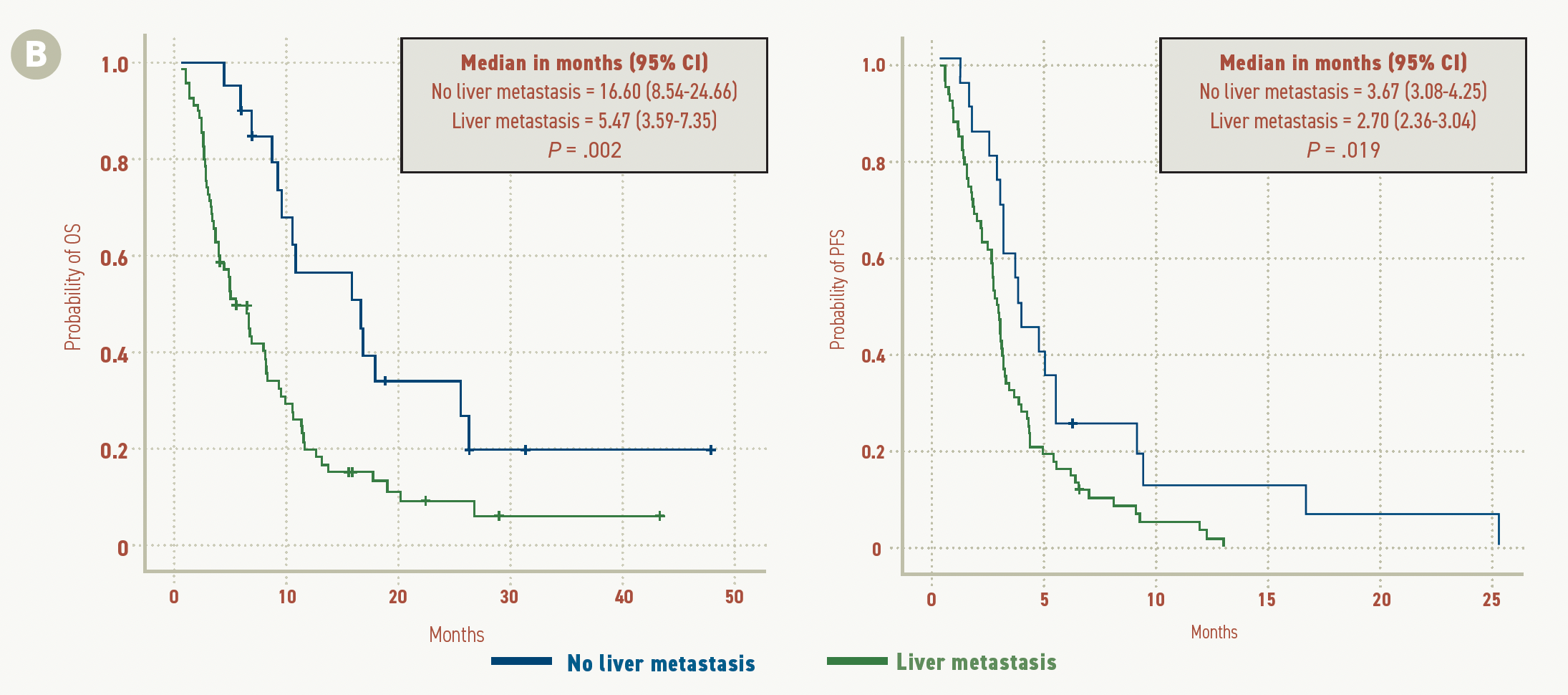
FIGURE 2C.
Effectiveness: OS and PFS
Survival analysis showed a median OS of 8.03 months (95% CI, 5.90-10.17) and a median PFS of 2.90 months (95% CI, 2.59-3.21). The OS and PFS curves are shown in Figure 1.
Median OS in patients with ECOG PS 0 or 1 was 9.50 months (95% CI, 6.62-12.38) vs 3.97 months (95% CI, 0.01-9.33) in patients with ECOG PS 2 or 3 (P = .017). PFS in those with ECOG PS 0 or 1 was 2.9 months (95% CI, 2.66-3.14) vs 2.5 months (95% CI, 1.81-3.19) in those with ECOG PS 2 or 3 (P = .282; Figure 2A).
The Kaplan-Meier curves showed that median OS was higher in the LTBS-low group than in the LTBS-med and LTBS-high groups, at 10.77 months (95% CI, 3.42-18.12) vs 6.77 months (95% CI, 2.25-11.29) vs 4.87 months (95% CI, 1.96-7.77; P = .003) respectively. Median PFS was also higher in LTBS-low patients at 3.67 months (95% CI, 2.67-4.66) vs 3.03 months (95% CI, 2.84-3.23) and 1.87 months (95% CI, 0.45-3.28) in the LTBS-med and LTBS-high groups, respectively (P = .004; Figure 2B).
Patients without liver metastasis had a median OS of 16.60 months (95% CI, 8.54-24.66) vs 5.47 months (95% CI, 3.59-7.35) in patients with liver metastasis (P = .002). A similar difference between patients with and without liver metastasis was also observed in median PFS, at 3.67 months (95% CI, 3.08-4.25) vs 2.70 months (95% CI, 2.36-3.04), respectively (P = .019; Figure 2C).
Safety
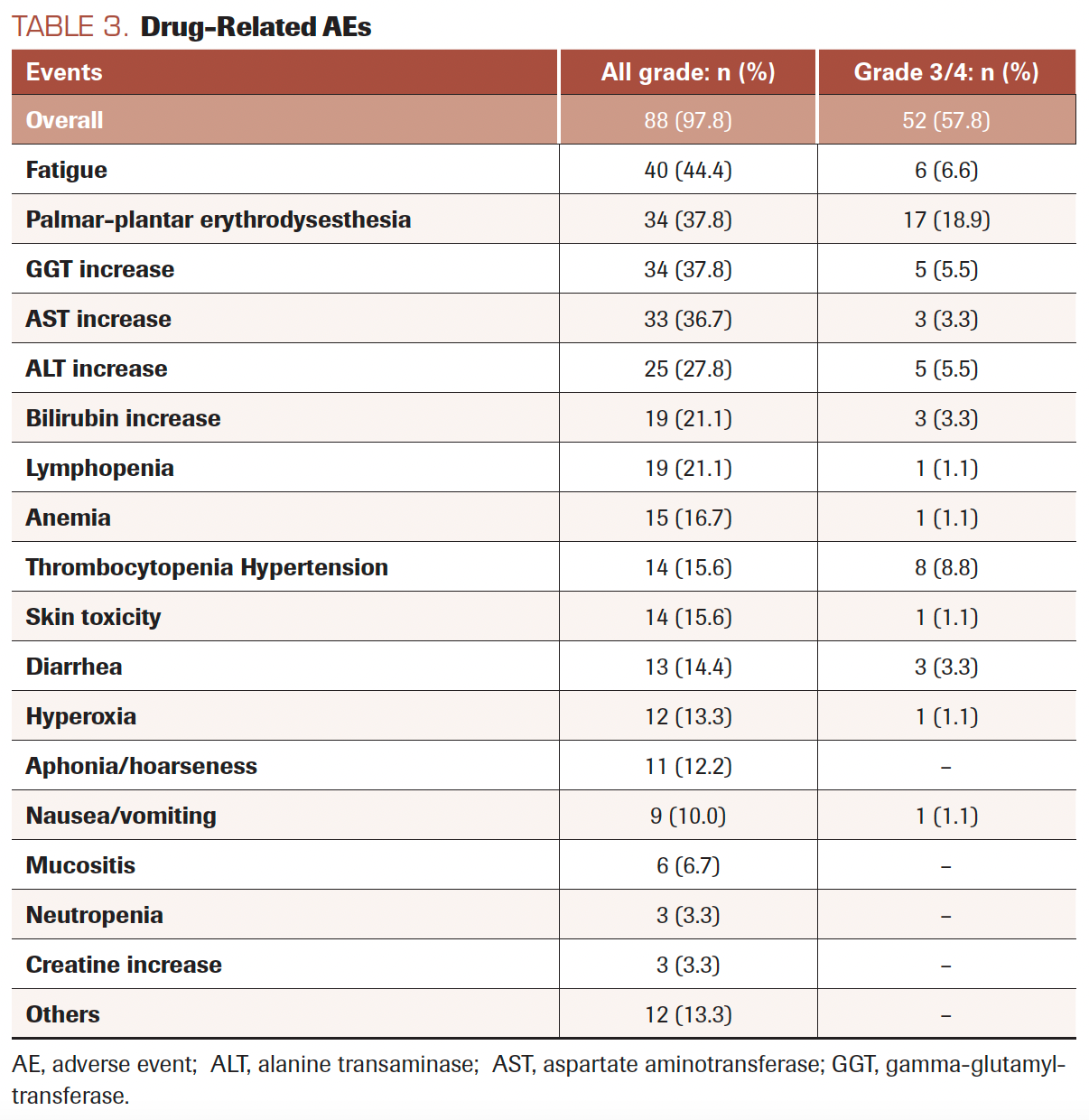
Eighty-eight patients (97.8%) experienced drug-related AEs during treatment. The most frequent were fatigue in 66 patients (73.3%), followed by palmar-plantar erythrodysesthesia in 40 (44.4%). Grade 3/4 drug-related AEs occurred in 52 patients (57.8%). Toxicity data are shown in Table 3. The dose of regorafenib had to be decreased due to toxicity in 37 patients (41.1%), of whom only 4 (11.8%) reached the maximum dose prior to the AE. Regorafenib treatment was discontinued in 30 patients (33.3%) and was interrupted in 29 (32.2%) due to drug-related AEs.
TABLE 3. Drug-Related AEs
Prognostic Factors for OS
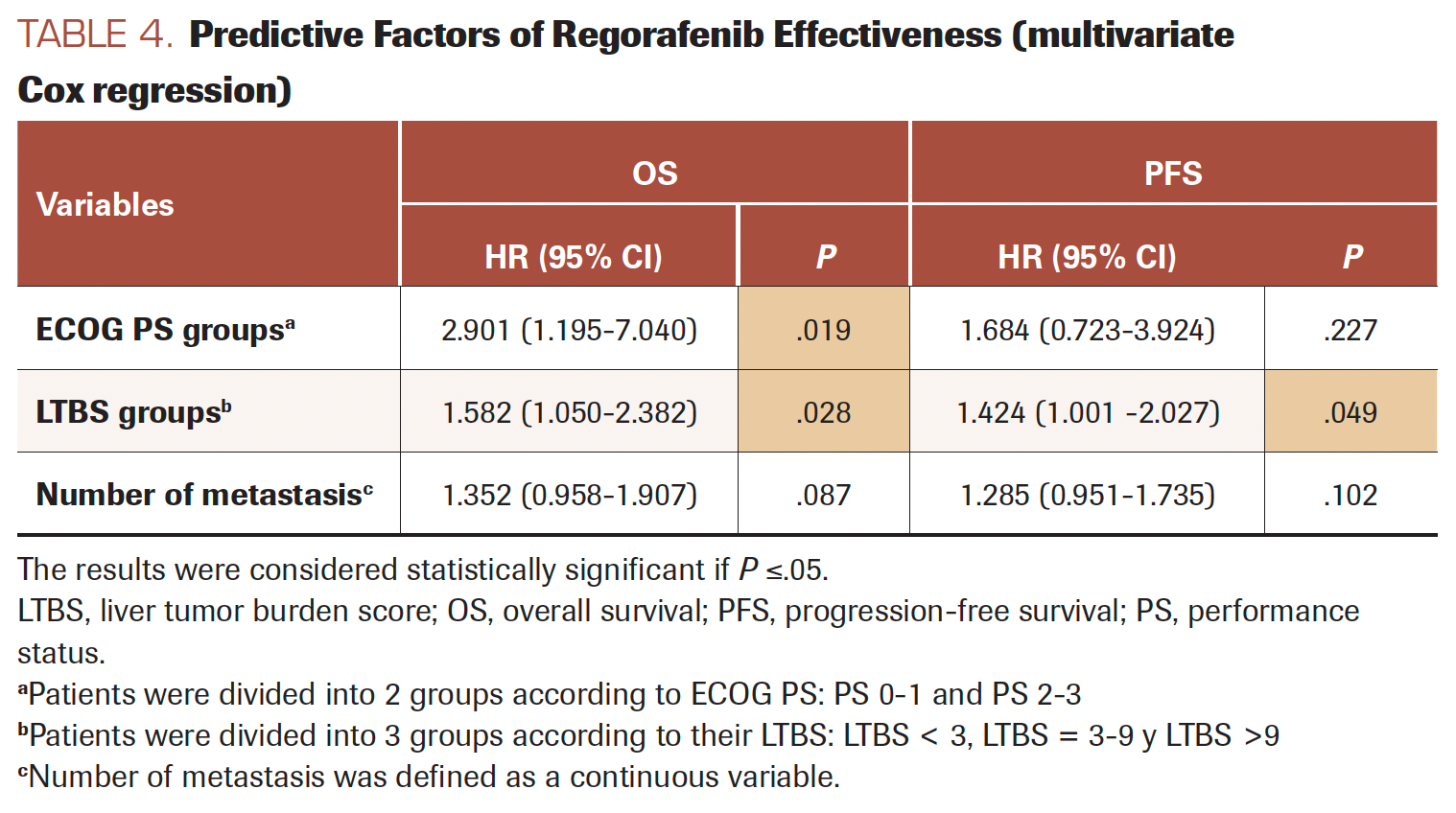
Univariate survival Cox regression analyses were performed (Supplement Table S1). Three variables were chosen for multivariate analysis based on their clinical relevance and our cohort size: ECOG PS, LTBS, and the number of metastases. The variable of liver metastasis was not included in the multivariate analysis because it interfered with LTBS (all patients without liver metastases had an LTBS value of 0). The results are shown in Table 4. The correlations of ECOG PS (HR, 2.901; 95% CI, 1.195-7.040; P =.019) and LTBS (HR, 1.582; 95% CI, 1.050-2-382; P = .028) to OS were statistically significant.
SUPPLEMENT TABLE S1. Univariate Cox Regression Analysis
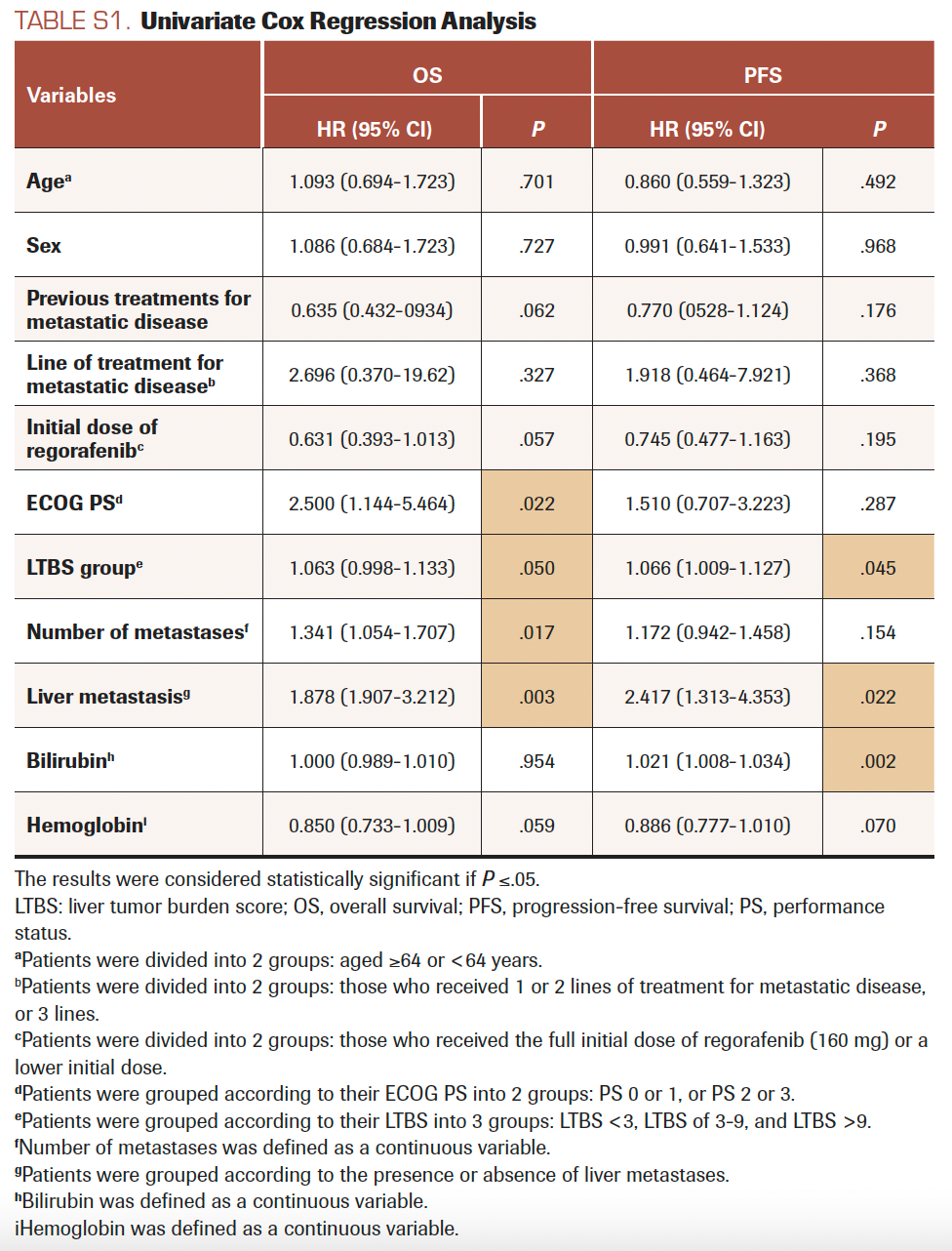
TABLE 4. Predictive Factors of Regorafenib Effectiveness (multivariate Cox regression)
Discussion
Regorafenib, an oral multikinase inhibitor of the tyrosine kinase receptor, is used to treat refractory mCRC. According to the ESMO-MCBS scale, regorafenib is considered a drug with low clinical benefit in metastatic disease.10 In clinical practice, regorafenib is often administered in patients with advanced, aggressive disease and impaired functional status, which might further limit its effectiveness. Therefore, we evaluated the routine clinical practice use of regorafenib and identified predictive factors of OS.
Our multicenter study included 90 patients. This study population had as many or more patients than many other retrospective studies14-17 but fewer than the studies led by Yamaguchi and Adenis.18,19 Almost half the patients in our study received regorafenib after 2 previous lines of treatment for metastatic disease, whereas the other half received it after 3 or more previous lines of therapy for metastatic disease. This fact might indicate that our study population was dominated by patients with refractory and aggressive disease. Most of the patients in our study had an ECOG PS of 0 or 1, as did most in the aforementioned pivotal trials8,9,20 and observational studies.19,21
The effectiveness of regorafenib was not affected by the number of previous lines of treatment in our cohort: OS was similar to what was reported in the CONCUR trial9 and higher than that reported in the CORRECT trial8 and in the REBECCA cohort evaluating regorafenib in earlier treatment lines.22,23
Regarding OS, the Kaplan-Meier analysis showed that patients with an ECOG PS of 0 or 1 achieved a median OS twice as long as that of patients with an ECOG PS of 2 or 3.18,21 Further, patients in the LTBS-low group had superior clinical benefit compared with patients in the LTBS-med group and especially compared with the LTBS-high group; there are currently no studies that analyze regorafenib survival based on LTBS with which we could compare these results. Finally, patients without liver metastases achieved a median OS 3 times higher than that of patients with liver metastases.
We found 2 predictive factors of OS benefit: the ECOG PS8,9,20,24 and LTBS. The number of metastases did not reach statistical significance in the multivariate analysis, but a positive trend was observed. A prior study which examined more patients than this one confirmed this variable as a predictor of higher OS.24 Therefore, we conclude that patients with an ECOG PS of 0 or 1, or who are LTBS-low and have no liver metastases, will benefit most from regorafenib treatment.
Like other tyrosine kinase inhibitors, regorafenib is associated with significant drug-related AEs, which can appear from the earliest cycles and impact the patient’s quality of life. AEs also affect treatment adherence, continuation of treatment, and dose reduction.25 In our study, nearly all patients reported some drug-related AEs—most frequently asthenia, followed by palmar-plantar erythrodysesthesia and liver enzyme alterations. These results show a higher incidence of drug-related AEs in routine clinical practice than in several prior clinical trials.8,9,20 In almost half the patients, dosage was reduced, and in one-third, treatment was discontinued or suspended due to drug-related AEs, similar to results reported by Rizzo and colleagues.26
In our cohort, almost half of all patients experienced grade 3/4 drug-related AEs, which could have a significant impact on quality of life and impaired functional status regardless. In addition, almost one-third of patients did not reach the full dose of 160 mg, which can alter efficacy. However, the ReDOS trial (NCT02368886) has shown that weekly dose escalation during the first cycle, from 80 mg to 160 mg daily, maintains the effectiveness (clinical outcomes) of the drug and reduces interruptions caused by drug-related AEs.27 All of these findings demonstrate that the toxicity profile of regorafenib is more complex in routine clinical practice28 than in phase 3 clinical trials.8,9
Limitations
This study had some limitations. As a retrospective analysis, it did not include a comparison cohort, and comparisons with other trials and studies were indirect. Furthermore, other studies have included larger sample sizes. Dose density duration was also not considered in the efficacy analysis.
Conclusions
In conclusion, in our study of routinely collected data of 90 patients with mCRC, treatment with regorafenib was associated with OS outcomes similar to those in randomized clinical trials and higher than those in some observational studies. Patients with ECOG PS of 0 or 1, LTBS lower than 3, and no liver metastases achieved higher OS. Regorafenib has low clinical benefit, but our data might be useful in identifying patients with mCRC who would be among the most likely to benefit from regorafenib treatment.
ACKNOWLEDGMENTS:
Statement of Ethics:
This study protocol was reviewed and approved by the Institutional Review Board (IRB) of La Princesa University Hospital. Approval number 4199.The study was granted an exemption from requiring written informed consent by the Institutional Review Board (IRB) of La Princesa University Hospital because of the retrospective character of the study.
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
Funding Sources:
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author Contributions:
Rebeca Mondéjar Solis, Berta Hernández Marin, Alberto Morell Baladrón, and Ramón Colomer Bosch contributed to the study conception and design. Material preparation and data collection were performed by Alberto Calvo-Garcia, Silvia Ruíz-García, Ana Beatriz Fernández Román, Javier Letellez Fernández, Beatriz Candel García, Carlos Hernández Terciado, Raquel De Santiago Álvarez, and Patricia Toquero Diez. Analysis was performed by Maria Pérez Abánades. The first draft of the manuscript was written by Alberto Calvo-Garcia and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability Statement:
The data sets generated during and/or analyzed during the current study are available from the corresponding author on request.
AUTHOR AFFILIATIONS:
Alberto Calvo-García, PharmG1, María Pérez Abánades, PharmG1, Silvia Ruíz-García, PharmG1, Ana Beatriz Fernández Román, PharmG2, Javier Letellez Fernández, PharmG2, Beatriz Candel García, PharmG2, Carlos Hernández Terciado, PharmG3, Raquel De Santiago Álvarez, PharmG3, Rebeca Mondéjar Solis, MD4, Berta Hernández Marin, MD4, Patricia Toquero Diez, MD4, Alberto Morell Baladrón, PharmG1, Ramón Colomer Bosch, MD4.
1Pharmacy Department, La Princesa University Hospital, Diego de León, 62, 28006, Madrid, Spain. +(34) 915202200.
2Pharmacy Department, Fuenlabrada University Hospital, Camino Del Molino, 2, 28942, Fuenlabrada (Madrid), Spain. +(34) 916006000.
3Pharmacy Department, Puerta de Hierro University Hospital, Calle Manuel de Falla, 1, 28222, Majadahonda (Madrid), Spain. +(34) 911916565.
4Oncology Department, La Princesa University Hospital, Diego de León, 62, 28006, Madrid, Spain. +(34) 915202200.
CORRESPONDING AUTHOR:
Alberto Calvo-García, PharmG
Pharmacy Department, La Princesa University Hospital
Diego de León, 62
28006, Madrid, Spain
Tel: +(34)915202200
Fax: +(34)915202235
Email: alberto.calvo@salud.madrid.org
REFERENCES
- Sideris M, Papagrigoriadis S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014;34(5):2061-2068.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Globocan 2020. Population Fact Sheet: United States of America. International Agency for Research on Cancer; 2020. Accessed October 2022. https://bit.ly/3TjVZoM
- Globocan 2020. Population Fact Sheet: Spain. International Agency for Research on Cancer; 2020. Accessed October 2022. https://bit.ly/3WUdQpd
- Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669-685. doi:10.1001/jama.2021.0106
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18(9):2658-2667. doi:10.1158/1078-0432.CCR-11-1900
- Food and Drug Administration. Stivarga prescribing information. Bayer (AG); 2013. Accessed October 2022. https://bit.ly/3DTkC5X
- Grothey A, Van Cutsem E, Sobrero A, et al; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. doi:10.1016/S0140-6736(12)61900-X
- Li J, Qin S, Xu R, et al; CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619-629. doi:10.1016/S1470-2045(15)70156-7
- ESMO-MCBS: ESMO-Magnitude of Clinical Benefit Scale. European Society for Medical Oncology; 2020. Accessed April 2021. https://bit.ly/3DCjtRz
- Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. U.S. Department of Health and Human Services; November 27, 2017. Accessed April 2021. https://bit.ly/3A61Ebf
- ECOG Performance Status Scale. ECOG-ACRIN Cancer Research Group. Accessed April 2021. https://bit.ly/3Dc3mtK
- Sasaki K, Morioka D, Conci S, et al. The Tumor Burden Score: a new “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267(1):132-141. doi:10.1097/SLA.0000000000002064
- Alawawdeh A, Price T, Karapetis C, et al. Regorafenib outcomes from the population based South Australian Metastatic Colorectal Cancer Registry. Asia Pac J Clin Oncol. 2022;18(4):428-433. doi:10.1111/ajco.13672
- Lam K-O, Lee K-C, Chiu J, et al. The real-world use of regorafenib for metastatic colorectal cancer: multicentre analysis of treatment pattern and outcomes in Hong Kong. Postgrad Med J. 2017;93(1101):395-400. doi:10.1136/postgradmedj-2016-134547
- Gotfrit J, Vickers M, Sud S, et al. Real-life treatment of metastatic colorectal cancer with regorafenib: a single-centre review. Curr Oncol. 2017;24(4):234-239. doi:10.3747/co.24.3562
- Wang R-T, Zhao Y, Wang A-L, Wang Y-T, Yin Z-P, Chen K. Efficacy and safety of regorafenib monotherapy among patients with previously treated metastatic colorectal cancer in a Chinese population: a real-world exploratory study. Int J Gen Med. 2021;14:5363-5373. doi:10.2147/IJGM.S325545
- Yamaguchi K, Komatsu Y, Satoh T, et al. Large-scale, prospective observational study of regorafenib in Japanese patients with metastatic colorectal cancer in a real-world clinical setting. Oncologist. 2019;24(7):e450-e457. doi:10.1634/theoncologist.2018-0377
- Adenis A, de la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer. 2016;16:412. doi:10.1186/s12885-016-2440-9
- Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncologist. 2019;24(2):185-192. doi:10.1634/theoncologist.2018-0072
- Mercier J, Voutsadakis IA. A systematic review and meta-analysis of retrospective series of regorafenib for treatment of metastatic colorectal cancer. Anticancer Res. 2017;37(11):5925-5934. doi:10.21873/anticanres.12039
- Carrato A, Benavides M, Massuti Sureda B, et al. Regorafenib (REG) as a single agent for first-line treatment of frail and/or unfit for polychemotherapy (PChT) patients (pts) with metastatic colorectal cancer (mCRC): a phase II study of the Spanish Cooperative Group for Digestive Tumor Therapy (TTD). J Clin Oncol.2016;34(Suppl 15):abstr 3527. doi:10.1200/JCO.2016.34.15_suppl.3527
- Del Prete M, Giampieri R, Loupakis F, et al. Prognostic clinical factors in pretreated colorectal cancer patients receiving regorafenib: implications for clinical management. Oncotarget. 2015;6(32):33982-33992. doi:10.18632/oncotarget.5053
- Moriwaki T, Fukuoka S, Masuishi T, et al. Prognostic scores for evaluating the survival benefit of regorafenib or trifluridine/tipiracil in patients with metastatic colorectal cancer: an exploratory analysis of the REGOTAS study. Int J Clin Oncol. 2020;25(4):614-621. doi:10.1007/s10147-019-01600-0
- Verbrugghe M, Duprez V, Beeckman D, et al. Factors influencing adherence in cancer patients taking oral tyrosine kinase inhibitors: a qualitative study. Cancer Nurs. 2016;39(2):153-162. doi:10.1097/NCC.0000000000000250
- Rizzo A, Nannini M, Novelli M, Ricci AD, Di Scioscio V, Abbondanza Pantaleo M. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920936932. doi:10.1177/1758835920936932
- Bekaii-Saab TS, Ou F-S, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070-1082. doi:10.1016/S1470-2045(19)30272-4
- O’Connor JM, Ducreux M, Petersen LN, et al; CORRELATE Investigators. Real-world dosing of regorafenib (REG) in metastatic colorectal cancer (mCRC): final results from the prospective, observational CORRELATE study. Ann Oncol. 2018;29(Suppl 8):VIII154-VIII155. doi:10.1093/annonc/mdy281.011
