Epidermal Growth Factor Receptor Inhibitors for the Treatment of Colorectal Cancer: A Promise Fulfilled?
The epidermal growth factor receptor (EGFR) is commonly expressedin colorectal cancers but not in most normal tissues, raising the possibilitythat this receptor could serve as a target for highly selective therapy.Based on preclinical studies demonstrating that antagonists of EGFRresulted in the inhibition of tumor growth, the development of clinicalreagents has been aggressively pursued. Early clinical studies demonstratedantitumor activity of EGFR inhibitors in patients with advancedcolorectal cancer, with acceptable toxicity. This early success fueledrapid clinical development. In this article, we will review the currentstatus of EGFR inhibitors in the treatment of patients with colorectalcancer, in an effort to describe both how far we have come as well aswhere we need to go in optimizing this promising therapeutic approach.
ABSTRACT: The epidermal growth factor receptor (EGFR) is commonly expressed in colorectal cancers but not in most normal tissues, raising the possibility that this receptor could serve as a target for highly selective therapy. Based on preclinical studies demonstrating that antagonists of EGFR resulted in the inhibition of tumor growth, the development of clinical reagents has been aggressively pursued. Early clinical studies demonstrated antitumor activity of EGFR inhibitors in patients with advanced colorectal cancer, with acceptable toxicity. This early success fueled rapid clinical development. In this article, we will review the current status of EGFR inhibitors in the treatment of patients with colorectal cancer, in an effort to describe both how far we have come as well as where we need to go in optimizing this promising therapeutic approach.
Stanley Cohen was awarded the Nobel Prize in Physiology or Medicine in 1986 for describing the epidermal growth factor (EGF) and its receptor (EGFR).[1] In 1962, he had initially observed that injection of submaxillary gland extracts induced precocious eyelid opening and tooth eruption in newborn mice.[2] Fifteen years later, the human homolog of the protein responsible for this activity, epidermal growth factor, and its receptor were isolated.[ 3-5] Cohen and colleagues subsequently demonstrated that this receptor had kinase activity, and that the binding and kinase activities resided in the same molecule.[6,7]
In the 1980s, studies demonstrated homology between EGFR and the proto-oncogene v-erbB, autocrine growth stimulation by TGF-alpha, and the fact that inhibition of EGFR with antibodies blocked phosphorylation, proliferation, and xenograft growth.[8-10] Also during the 1980s, several reports indicated that approximately one-third of all human epithelial cancers overexpress EGFR, and that this expression is an indicator of poor prognosis.[11-16] These findings suggested that EGFR might serve as a target for anticancer therapy.
Forty years after an unexpected laboratory observation, clinical reagents have been developed and have resulted in tumor regression in patients with colorectal and other cancers. This review will consider the compounds furthest along in clinical development against colorectal cancer, in an effort to elucidate not only how far we have come, but how far we still need to go in optimizing the use of EGFR antagonists.
Epidermal Growth Factor Receptor
EGFR (aka erbB1 or HER1) is a ubiquitous 170-kd transmembrane glycoprotein. The cell surface contains the amino-terminal glycosylated extracellular ligand-binding domain. Embedded in the cell membrane is a short, helical, hydrophobic transmembrane region, contiguous to the intracellular carboxy-terminal cytoplasmic domain that contains the tyrosine kinase domain.[17,18] Signaling by the EGFR is critical for the fetal development of epithelial, neuronal and mesenchymal tissue. Mice that lack EGFR have abnormal development of the lungs, gastrointestinal tract, skin, liver, eyes, and brain.[19-21] In the adult, EGFR signaling is tightly controlled. EGFR is a critical component of wound healing and normal cell signaling.
FIGURE 1
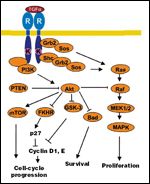
Mechanisms of Receptor Activation
EGFR activation is initiated by the binding of a ligand-such as EGF, transforming growth factor (TGF)- alpha, amphiregulin, heparin-binding EGF, betacellulin, or epiregulin-to the extracellular domain. The bound receptor then homodimerizes, with phosphorylation of the tyrosine kinase domain and the subsequent cascade of signaling pathways that transmits the message to the nucleus. EGFR activates several intracellular signaling pathways including phosphatidylinositol- 3 kinase (PI3K)-Akt, Ras-Raf- MEK-MAPK, Src, PLC-gamma-1, and PAK-JNKK-JNK (Figure 1).[22,23] Successful transmission of the message results in cell division, differentiation, migration, and protection from apoptosis.[24] The extracellular receptor is then internalized through endocytosis, with subsequent receptor degradation or recycling to the cell surface.
Rationale for EGFR as Therapeutic Target
Overexpression of EGFR has been documented in a variety of human cancers, including gliomas and carcinomas of the kidney, bladder, breast, ovary, pancreas, lung, rectum, and colon.[25-27] The EGFR is present in approximately 25% to 77% of all colorectal malignancies, depending on the method of measurement.[27] In some studies, higher levels of EGFR expression correlate with more aggressive clinical behavior. Patients with tumors that overexpress EGFR tend to have a worse prognosis with shorter survival and increased risk of metastases.[11,12,14-16,28-33]
For example, Khorana et al[34] recently reported a study of EGFR expression in 131 consecutive patients who underwent surgery for stage II/III primary colon cancers. Of 131 tumors, 60 (46%) had EGFR expression by immunohistochemistry (graded 1-3), while the surrounding normal colonic mucosa did not. Five-year survival in patients with no or grade 1 EGFR expression was 55%, with a median survival of 5.5 years (95% confidence interval [CI] = 4.1-8.9 years). In patients with grade 2 or 3 EGFR expression, 5-year survival was 48%, with a median survival of 4.5 years (P = .05). These data suggest that overexpression of EGFR in patients with stage II/III colorectal cancer is associated with inferior survival. Preclinical studies have also shown that cells overexpressing EGFR tend to exhibit enhanced drug and radiotherapy resistance.[35,36]
Uncontrolled EGFR activation results in uncontrolled cellular growth and may therefore be pathogenetic for some tumors. Potential causes of excessive receptor activation include mutations of the receptor or any of its domains (constitutive activation), increased ligand concentration, and decreased receptor degradation. The possibility that EGFR expression is a marker for activation that is driving some tumors led to the exploration of inhibitors of this pathway as cancer therapeutic agents. Many preclinical studies validated this hypothesis.[ 10,37-41] Given that EGFR expression is common in colorectal cancers, this setting provides a natural context in which to explore the clinical utility of the approach.
Approaches to EGFR Blockade
Several approaches to targeting EGFR are being pursued in the clinic. The furthest along in development are antibodies to the extracellular domain and small-molecular-weight tyrosine kinase inhibitors. The high target selectivity of these agents initially generated great optimism that they would provide a superior therapeutic index when compared with traditional cytotoxics and could be readily combined with other agents or modalities. In cell culture and xenograft models, antibodies and small molecules have similar effects in inhibiting the cell cycle, promoting apoptosis, and inhibiting angiogenesis.[42-49] Several distinguishing biologic features are outlined in Table 1.
TABLE 1

Comparison of EGFR Antibodies and Small-Molecule Tyrosine Kinase Inhibitors
In addition to EGFR antibodies and small-molecule tyrosine kinase inhibitors, other approaches to blocking EGFR activity are also in development. These include anti-EGF vaccines, immunoconjugates, multifunctional antibodies, and antisense oligonucleotides. These compounds are in early clinical trials, and will not be addressed further in this review.
Preclinical Data
Study of the EGFR pathway, including consequences of inhibition, was dramatically accelerated in the late 1970s by the identification of the A431 squamous cancer cell line, which has abundant expression of EGFR. Preclinical results obtained with A431 cells and similar lines provided the basis for the clinical development of EGFR inhibitors. The use of cell lines with extremely dense EGFR expression that are clearly dependent on this pathway for growth was critical to inhibitor development but may have contributed to an expectation that human cancers would routinely respond similarly to EGFR inhibition in vivo. As described below, this has not been the case, suggesting that EGFR expression may be necessary but not sufficient for antitumor activity.
Anti-EGFR monoclonal antibodies (eg, cetuximab [Erbitux], ABXEGF, EMD 72000) are designed to bind exclusively to the extracellular domain of EGFR.[50,51] This, in turn, inhibits the binding of ligands (such as EGF or TGF-alpha) to EGFR, and, hence, inhibits subsequent signal transduction. The receptor-antibody complex is ultimately internalized and degraded, resulting in receptor cell surface downregulation.[50] In contrast, small-molecule inhibitors first enter the cell and then bind the receptor's tyrosine kinase domain, thereby inhibiting downstream signaling.
In cell culture and xenograft models, EGFR inhibition, regardless of method, has several consistent effects. These include (1) inhibition of angiogenesis (via downregulation of vascular endothelial growth factor)[45,52] and reduced expression of molecules associated with invasion and metastasis (eg, MMP-9, IL-8)[53,54]; (2) promotion of apoptosis[ 44,55]; (3) cell-cycle inhibition with inhibition of proliferation[44]; and (4) potentiation of radiation and chemotherapy sensitivity.[37,38,56] This last observation in particular has guided the early clinical development of EGFR inhibitors; whereas, traditionally, single-agent activity is defined prior to combination studies, this paradigm has not been routinely employed with EGFR inhibitors (see Clinical Development section, which follows).
Clinical Development
Antibodies
• Cetuximab-Cetuximab is a chimeric immunoglobulin (Ig)G1 monoclonal antibody EGFR inhibitor. It binds the EGFR with high affinity (dissociation constant Kd = 2.0 * 10-10 M), 1 log higher than the natural ligand. This agent is under clinical development in a variety of malignancies including colorectal cancer, head and neck cancer, ovarian cancer, and pancreatic cancer.
The phase I experience with cetuximab established saturable kinetics consistent with EGFR internalization as a primary mechanism of elimination. A weekly dose for phase II testing was chosen based on a long half-life (days) and routine achievement of trough levels that exceeded receptor saturation in xenograft models.[ 57] The predominant toxicity, skin rash, was not dose-limiting at the selected phase II dose. Given colorectal xenograft models suggesting potentiation of cetuximab activity when administered with irinotecan (Camptosar), and the fact that complete tumor growth inhibition was only achieved with the combination,[39] the initial phase II study in colorectal cancer tested cetuximab plus irinotecan.
This initial study in patients with colorectal cancer evaluated the combination of cetuximab plus irinotecan in patients with EGFR-positive (≥ 1+ by immunohistochemistry) metastatic disease that had progressed on or shortly after previous irinotecan therapy.[ 58] A small cohort of patients who had stable disease on irinotecan was also included. Cetuximab was given with a loading dose followed by weekly treatment, and irinotecan was administered as in the previously used schedule-either every 3 weeks or weekly (for 4 of 6 weeks). In this study, 120 patients were enrolled in the "refractory" cohort and 18 in the "stable" cohort. In a preliminary report, a 22.5% response rate (95% CI = 15%-31%) was described in the 120 patients with irinotecanrefractory, EGFR-positive colorectal cancer, and a 44% response rate was observed in patients with previously stable disease.
TABLE 2

Survival and Response as a Function of Skin Rash Severity in Chemotherapy-Refractory Metastatic Colorectal Cancer Patients Treated With Cetuximab and Irinotecan
Time to tumor progression in responding patients was 186 days in the refractory cohort.[58] The treatment was well tolerated, with a sterile folliculitis- type skin rash the most common toxicity. Of note, the investigators observed an association among skin rash severity, response, and survival (Table 2). This suggests the possibility that skin may serve as a surrogate for response to EGFRinhibition therapy. On the other hand, the degree of EGFR-staining intensity as evaluated by immunohistochemistry did not correlate with response.
One question raised by these data concerns whether the inclusion of irinotecan contributed to the clinical activity observed. In an effort to shed light on this issue, a cetuximab monotherapy study was conducted in a similar population of EGFR-positive colorectal cancer patients who had failed previous therapy with irinotecan. Saltz et al[59] presented the preliminary results of this phase II trial of singleagent cetuximab in 57 patients with metastatic colorectal cancer. Patients were treated with cetuximab at standard doses (400-mg/m2 loading dose over 2 hours, then 250 mg/m2 over 1 hour weekly). The most commonly encountered adverse events were an acne-like skin rash, predominantly on the face and upper torso (86% of patients with any grade, 16% with grade 3) and asthenia (53% any grade, 7% grade 3). Two patients (3.5%) experienced grade 3 allergic reactions requiring discontinuation of study treatment. Six patients (11%, 95% CI = 4%-22%) achieved a partial response. An additional 13 patients had radiographically stable disease. This study established the single-agent activity of cetuximab.
Further data confirming the two studies described above was reported at the May 2003 meeting of the American Society of Clinical Oncology by Cunningham et al.[60] In this randomized phase II trial, 329 patients with EGFR-positive, irinotecanrefractory metastatic colorectal cancer were given either cetuximab alone or cetuximab in combination with irinotecan. In a 2:1 randomization, 218 patients received the combination, and 111 patients received cetuximab monotherapy. The intent-to-treat analysis showed a response rate of 10.8% (95% CI = 5.7%-18.1%) for cetuximab alone, and 22.9% (95% CI = 17.5%-29.1%) for cetuximab in combination with irinotecan (P = .0074). The median time to progression was 1.5 months for cetuximab alone and 4.1 months for the combination (P ≤ .0001). However, there was no difference between the arms in median overall survival (6.9 vs. 8.6 months, P = .48).
It is important to note that this study was not designed or powered as a phase III comparison. Serious events observed were consistent with the known safety profiles of irinotecan and cetuximab, with reversible skin toxicity and allergic reactions directly attributable to cetuximab. The results of this study show response rates similar to those reported in the other phase II colon cancer trials, and lend support to the hypothesis that response and survival may be improved when irinotecan is administered along with cetuximab. Given that the addition of irinotecan contributes additional toxicity, the decision of whether to treat an individual patient with monotherapy or combination therapy will ultimately require the integration of patient preferences and values with objective antitumor response data from clinical trials. The Cunningham study also provided additional data supporting the association of skin rash with antitumor activity (Table 3).
TABLE 3

Correlation of Efficacy and Skin Toxicity in Patients With EGFR-Positive, Irinotecan-Refractory Metastatic Colorectal Cancer
Cetuximab is also being explored as a component of first-line therapy against metastatic colorectal cancer. Preliminary phase II data have been reported from three studies combining cetuximab with irinotecan, fluorouracil (5-FU), and leucovorin in previously untreated patients with EGFR-expressing metastatic colorectal cancer. At ASCO 2002, Schoffski et al[61] presented early data from a study of cetuximab in combination with a weekly regimen of irinotecan and infusional 5-FU and leucovorin. Partial responses were documented in four of six evaluable patients at the time of abstract submission.
Rosenberg et al[62] presented preliminary data from a phase II study of cetuximab with weekly irinotecan, bolus 5-FU, and leucovorin in metastatic colorectal cancer patients, 11 (44%, 95% CI = 24%-68%) of whom achieved a partial response. An additional five patients achieved a minor response (> 40% reduction in tumor size). At ASCO 2003, Van Laethem et al also presented preliminary safety and efficacy results from a phase II trial of cetuximab in combination with FOLFIRI (biweekly infusional 5-FU and irinotecan).[63] Of 18 patients evaluable for efficacy to date, 12 (67%) had a partial response and 4 (22%) had stable disease.
These three trials are leading to phase III licensing studies for cetuximab in front-line therapy. Additional studies combining cetuximab with oxaliplatin (Eloxatin)-based regimens are also under way. In all studies, the toxicities associated with cetuximab have been generally well tolerated. As with other EGFR inhibitors, a sterile, suppurative folliculitis (acne-like rash) presenting predominantly on the face, scalp, chest, and upper back is common but usually not dose-limiting. When combined with chemotherapy, cetuximab does not appear to augment expected cytotoxic side effects. Significant hypersensitivity reactions occur in less than 5% of patients.
Overall, cetuximab is a well-tolerated drug with clear activity in patients with metastatic colorectal cancer. Ongoing research will help define its role in first-line and subsequent therapy, as well as in the adjuvant setting. The clinical data on irinotecan to date suggest that superior response rates will be seen in combination with other active cytotoxic agents. As noted above, if these data are confirmed, clinical decision-making for individual patients will need to integrate consideration of toxicity as well as antitumor activity.
• ABX-EGF-ABX-EGF is a highaffinity (Kd = 5.0 * 10-11), fully human IgG2 monoclonal antibody directed at EGFR.[64] Preclinical trials in xenograft models suggested that ABX-EGF might have activity against colorectal cancer.[65] A phase I study of weekly treatment demonstrated a prolonged half-life (days) and no doselimiting toxicities at dosages that produced trough concentrations necessary to achieve receptor saturation in preclinical models.[66] As with cetuximab, clearance is saturable. The dose selected for initial phase II testing (2.5 mg/kg weekly) is predicted to result in skin rash in nearly all patients.[67]
Meropol et al[68] reported a phase II trial of ABX-EGF as monotherapy in patients with metastatic colorectal cancer. Forty-four previously treated patients with metastatic colorectal cancer and +2 or +3 EGFR expression in > 10% of tumor cells were treated with ABX-EGF, 2.5 mg/kg/wk. All patients had failed fluoropyrimidine therapy, as well as irinotecan and/or oxaliplatin. A planned interim analysis documented 4 partial responses in 40 evaluable patients (10%), and an additional 22 (55%) with stable disease. All 44 patients had a skin rash, with the maximal skin rash intensity generally reached by week 3 of therapy. Three patients had grade 3 skin rash. Other severe adverse events were infrequent.
Given that ABX-EGF is a fully human antibody, allergic reactions were not anticipated and did not occur in the cohort reported. This study is ongoing, with a planned accrual of 100 patients in the initial cohort; an additional 50 patients with tumors expressing EGFR in at least 10% of cells, but less than 10% 2+ to 3+, were also subsequently accrued. Results in these additional patients are pending.
• EMD 72000-EMD 72000 is a humanized monoclonal antibody that binds to the extracellular domain of EGFR and shows preclinical activity in human tumor xenografts.[69] Two phase I monotherapy trials in patients with EGFR-positive tumors have been reported. In the initial study,[70] EMD 72000 was administered weekly in escalating doses between 400 and 2,000 mg. The maximum tolerated dose was 1,600 mg per week, with headache and fever dose-limiting at 2,000 mg weekly. Follicular rash was the most common adverse event reported but was not dose-limiting. Eleven patients with metastatic colorectal cancer were treated in this study, and two partial responses were reported. Pharmacokinetics were dose proportional, with a prolonged halflife and drug accumulation over time suggesting the possibility of less frequent dosing.
Tabernero et al[71] recently presented results of the other phase I clinical trial of EMD 72000, in which 1-, 2-, and 3-week dosing intervals were explored. In addition, pre- and posttreatment skin and tumor biopsies were performed for in vivo pharmacodynamic assessment. A total of 27 patients were enrolled, 19 of whom had colorectal cancer. Partial responses were documented in three of the patients with colorectal cancer, two of whom received 1,200 mg weekly, and one of whom was treated with 1,200 mg every 3 weeks. Preliminary pharmacodynamic analyses of skin biopsies showed posttreatment decreases in phosphorylated EGFR, phosphorylated MAP kinase, and Ki67, with an increase in p27. Likewise, preliminary analyses of tumor biopsies confirmed decreases in phosphorylated EGFR, phosphorylated MAP kinase, and phosphorylated AKT with treatment.
These early data demonstrate downstream effects of EGFR blockade as predicted in preclinical models. Further analyses of pharmacokinetic and pharmacodynamic end points on this study will help guide the selection of dose and interval for phase II studies in colorectal and other diseases.
TABLE 4

Comparison of Anti-EGFR Antibodies
Features of the anti-EGFR monoclonal antibodies discussed above are summarized in Table 4. Each of these agents has shown clinical activity against colorectal cancer. Whether their differences in structure will impact clinical utility has yet to be determined. Current data suggest that there may ultimately be differences in administration schedule and toxicities (eg, frequency and intensity of skin rash, hypersensitivity reactions). Whereas cetuximab and EMD 72000 can participate in antibody-dependent cellular cytotoxicity (based upon their IgG1 backbone), ABX-EGF (IgG2) cannot. Furthermore, these agents differ in their affinity for EGFR. The impact of these differences on mechanism of action, pharmacodynamics, and clinical activity is yet to be determined.
Small Molecules
Several small-molecule EGFR tyrosine kinase inhibitors have entered clinical trials in patients with metastatic colorectal cancer. In contrast to EGFR antibodies, the small molecules are administered orally on a daily basis. As noted above, these agents competitively occupy the ATP-binding pocket on the intracellular domain of the EGFR, thereby blocking downstream signaling. Although their effects are similar to those observed with EGFR antibodies in preclinical models, and although they have shown single-agent activity in patients with lung and other cancers, current data suggest that the small-molecule EGFR inhibitors are relatively inactive in patients with colorectal cancer.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
ABX-EGF
Capecitabine (Xeloda)
Cetuximab (Erbitux)
EMD 72000
Erlotinib (Tarceva)
Fluorouracil (5-FU)
Gefitinib (Iressa)
Irinotecan (Camptosar)
Mitomycin (Mutamycin)
Oxaliplatin (Eloxatin)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
• Gefitinib-Gefitinib (Iressa) is currently licensed for use in patients with non-small-cell lung cancer who have failed chemotherapy, based on monotherapy response rates of 10% to 20%.[72,73] Several phase II studies of gefitinib in patients with metastatic colorectal cancer have been reported.
Goss et al[74] reported a phase I/II study of 27 patients who had refractory colorectal cancer and were treated with gefitinib. No objective responses were confirmed. Dorligschaw et al[75] studied gefitinib, 250 mg/d, alone or in combination with chemotherapy (capecitabine [Xeloda]/irinotecan, capecitabine/mitomycin [Mutamycin], capecitabine/oxaliplatin, irinotecan, or capecitabine) as a "last-line" option in heavily pretreated patients with advanced colorectal cancer. Of 14 patients, 10 were evaluable for efficacy. No responses were documented.
A phase II study by the Eastern Cooperative Oncology Group (ECOG 6200) is evaluating two different doses of gefitinib in patients with metastatic colorectal cancer. Accrual to this trial is complete, and results are pending. In addition to tumor response, serum levels of the EGFR ligands TGF-alpha and amphiregulin as well as effects of gefitinib on intratumoral EGF, MAP kinase, AKT, and Ki67 will be evaluated before and after treatment.
Although gefitinib appears to be inactive as a single agent against metastatic colorectal cancer, preclinical and clinical data suggest the potential for synergy with cytotoxic drugs. Braun et al[76] studied the effect of gefitinib on the cellular determinants of resistance to the active metabolite of irinotecan (SN-38) in drug-sensitive (HCT-8/wt) and fourfold SN-38- resistant (HCT-8/SN-38) human colon cancer cells. These studies suggested that gefitinib-mediated inhibition of EGFR tyrosine kinase can reverse resistance to SN-38 in human colon tumor cells.
Cho et al[77] reported preliminary clinical evidence of a cooperative effect of gefitinib and cytotoxic chemotherapy in a study of gefitinib in combination with FOLFOX-4 (infusional 5-FU, leucovorin, oxaliplatin) in patients with advanced colorectal cancer. Of 12 patients who received this combination in the first-line setting, 9 (75%) had a documented partial response, while 3 (23%) of 13 patients who received FOLFOX plus gefitinib as a second or greater line of therapy had a documented partial response. This study is ongoing.
• Erlotinib-Erlotinib (Tarceva) is another orally administered smallmolecule EGFR tyrosine kinase inhibitor.[ 78] This agent has shown clinical activity against non-small-cell lung cancer,[79] head and neck cancer,[ 80] and ovarian cancer,[81] and in preclinical studies, activity against primary colon cancer cells has been reported.[82] Unfortunately, a phase II study in patients with metastatic colorectal cancer showed no partial or complete responses.[83]
As with the EGFR antibodies, the predominant side effect of small-molecule tyrosine kinase inhibitors has been skin rash. Diarrhea has also been reported. The apparent lack of clinical activity of small-molecule EGFR inhibitors in colorectal cancer has been somewhat surprising given preclinical activity similar to that observed with antibodies, in vivo demonstration of target inhibition and downstream effects in skin biopsies,[84-87] and clear clinical activity against other malignancies. It is plausible that the pharmacology (including drug penetration at tumor sites), and pharmacodynamics (eg, target inhibition and on/ off rates associated with small molecules) are distinguishing features that result in meaningful clinical differences between these classes of agents.
Conclusions
Drugs that target the EGFR pathway constitute an excellent example of successes and pitfalls inherent in the development of highly selective, targeted therapies against cancer. For an ideal selective, targeted therapy, the target would be expressed preferentially in malignant cells and not in normal cells. The target would contribute significantly to the malignant phenotype, and the inhibition of the target would reverse the phenotype. The presence or absence of the target would be measurable so that only patients with tumors that have the target are treated with the targeting agent. Finally, the toxicity to normal tissues should not be excessive.
Several of these criteria are clearly met for the EGFR inhibitors, including an excellent therapeutic index and selective target expression in malignant tissues. However, only a minority of colorectal tumors responds to EGFR blockade with antibodies, and small molecules are ineffective as single agents. The greatest challenge for clinical investigators is to better define which patients should be treated with these agents, such that we can enrich the population of treated patients with responders. At this point, there has been no association between EGFR expression by immunohistochemistry and antitumor activity. This suggests that our current measure of target expression is inadequate. In other words, target expression by immunohistochemistry does not indicate which tumors are actually driven by or dependent on the EGFR pathway.
TABLE 5

Potential Influences on Response to EGFR Inhibitors
Several potential influences on response to EGFR inhibition exist (Table 5). The elucidation of factors influencing target expression, natural ligand expression, ligand binding, and phosphatase activity is under way. For example, genetic polymorphisms in EGFR may affect gene expression[ 88,89] and ligand binding.[90] Dimerization or association of EGFR with other growth factor receptors or pathways could also affect response to selective inhibitors of EGFR. It is also likely that downstream interaction of EGFR signal transducers with other cellular pathways defines the dependence of tumors on EGFR signaling.
As described above, several efforts have been undertaken to define the pharmacodynamic effects of EGFR inhibition in vivo. These ambitious studies are critical to defining the factors that will ultimately comprise a "response profile" for EGFR inhibitors. Although clinical response data suggest that the measurement of pharmacodynamics in skin may have predictive value, the continuation of studies requiring tumor biopsies, while challenging, are critical in validating such surrogates. Further clinical trials will also determine if dosing targeted to the development of skin rash can improve antitumor response. Development of less invasive methods of in vivo pharmacodynamic assessment, such as isolation and characterization of tumor cells in the circulation, are under way.[91] In addition, gene expression studies are being conducted in an effort to identify signatures of response and resistance.
Over the past few years, the clinical development of EGFR inhibitors in colorectal cancer has been rapid. Proof of principle has been obtained for the activity of inhibitory antibodies, leading to justified excitement among scientists, clinicians, and patients. However, several remaining challenges will require a partnership between clinical and laboratory investigators, as well as academia and industry. As current studies address the activity of monoclonal antibodies in combination with cytotoxics in advanced disease and the adjuvant setting, ongoing correlative studies must continue to explore questions related to mechanism of action, dose and schedule optimization, and patient and tumor characteristics predictive of response and toxicity. These explorations will also lead to the development of additional rational approaches to targeting the EGFR pathway. An odyssey that began more than 40 years ago still has far to go.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Weltman JK: The 1986 Nobel Prize forPhysiology or Medicine awarded for discoveryof growth factors: Rita Levi-Montalcini,M.D., and Stanley Cohen, Ph.D. N Engl RegAllergy Proc 8:47-48, 1987.
2.
Cohen S: Isolation of a mouse submaxillarygland protein accelerating incisor eruptionand eyelid opening in the new-born animal. JBiol Chem 237:1555-1562, 1962.
3.
Taylor JM, Mitchell WM, Cohen S: Epidermalgrowth factor. Physical and chemicalproperties. J Biol Chem 247:5928-5934, 1972.
4.
Starkey RH, Cohen S, Orth DN: Epidermalgrowth factor: Identification of a new hormonein human urine. Science 189:800-802,1975.
5.
Cohen S, Carpenter G: Human epidermalgrowth factor: Isolation and chemical and biologicalproperties. Proc Natl Acad Sci U S A72:1317-1321, 1975.
6.
Carpenter G, King L Jr, Cohen S: Epidermalgrowth factor stimulates phosphorylationin membrane preparations in vitro. Nature276:409-410, 1978.
7.
Cohen S, Carpenter G, King L Jr: Epidermalgrowth factor-receptor-protein kinase interactions.Co-purification of receptor and epidermalgrowth factor-enhanced phosphorylationactivity. J Biol Chem 255:4834-4842,1980.
8.
Downward J, Yarden Y, Mayes E, et al:Close similarity of epidermal growth factorreceptor and v-erb-B oncogene protein sequences.Nature 307:521-527, 1984.
9.
Downward J, Parker P, Waterfield MD:Autophosphorylation sites on the epidermalgrowth factor receptor. Nature 311:483-485,1984.
10.
Goldstein NI, Prewett M, Zuklys K, etal: Biological efficacy of a chimeric antibodyto the epidermal growth factor receptor in ahuman tumor xenograft model. Clin CancerRes 1:1311-1318, 1995.
11.
Harris AL, Nicholson S, Sainsbury JR,et al: Epidermal growth factor receptors inbreast cancer: Association with early relapseand death, poor response to hormones and interactionswith neu. J Steroid Biochem 34:123-131, 1989.
12.
Hemming AW, Davis NL, Kluftinger A,et al: Prognostic markers of colorectal cancer:An evaluation of dna content, epidermal growthfactor receptor, and Ki-67. J Surg Oncol51:147-152, 1992.
13.
Etienne MC, Formento JL, Lebrun-Frenay C, et al: Epidermal growth factor receptorand labeling index are independent prognosticfactors in glial tumor outcome. ClinCancer Res 4:2383-2390, 1998.
14.
Klijn JG, Berns PM, Schmitz PI, et al:The clinical significance of epidermal growthfactor receptor (EGF-R) in human breast cancer:A review on 5232 patients. Endocr Rev13:3-17, 1992.
15.
Ozanne B, Richards CS, Hendler F, etal: Over-expression of the EGF receptor is ahallmark of squamous cell carcinomas. J Pathol149:9-14, 1986.
16.
Yamanaka Y, Friess H, Kobrin MS, et al:Coexpression of epidermal growth factor receptorand ligands in human pancreatic canceris associated with enhanced tumor aggressiveness.Anticancer Res 13:565-569, 1993.
17.
Schlessinger J: Cell signaling by receptortyrosine kinases. Cell 103:211-225, 2000.18. Yarden Y: The EGFR family and itsligands in human cancer. Signalling mechanismsand therapeutic opportunities. Eur JCancer 37(suppl 4):S3-S8, 2001.
19.
Threadgill DW, Dlugosz AA, HansenLA, et al: Targeted disruption of mouse EGFreceptor: Effect of genetic background on mutantphenotype. Science 269:230-234, 1995.
20.
Miettinen PJ, Berger JE, Meneses J, etal: Epithelial immaturity and multiorgan failurein mice lacking epidermal growth factorreceptor. Nature 376:337-341, 1995.
21.
Sibilia M, Steinbach JP, Stingl L, et al:Strain-independent postnatal neurodegenerationin mice lacking the EGF receptor.EMBO J 17:719-731, 1998.
22.
Arteaga CL: The epidermal growth factorreceptor: From mutant oncogene in nonhumancancers to therapeutic target in humanneoplasia. J Clin Oncol 19(18 suppl):32S-40S,2001.
23.
Mendelsohn J: Targeting the epidermalgrowth factor receptor for cancer therapy. J ClinOncol 20(18 suppl):1S-13S, 2002.
24.
Hackel PO, Zwick E, Prenzel N, et al:Epidermal growth factor receptors: Criticalmediators of multiple receptor pathways. CurrOpin Cell Biol 11:184-189, 1999.
25.
Ekstrand AJ, James CD, Cavenee WK,et al: Genes for epidermal growth factor receptor,transforming growth factor alpha, and epidermalgrowth factor and their expression inhuman gliomas in vivo. Cancer Res 51:2164-2172, 1991.
26.
Moscatello DK, Holgado-Madruga M,Godwin AK, et al: Frequent expression of amutant epidermal growth factor receptor inmultiple human tumors. Cancer Res 55:5536-5539, 1995.
27.
Salomon DS, Brandt R, Ciardiello F, etal: Epidermal growth factor-related peptidesand their receptors in human malignancies. CritRev Oncol Hematol 19:183-232, 1995.
28.
Grandis JR, Tweardy DJ, Melhem MF:Asynchronous modulation of transforminggrowth factor alpha and epidermal growth factorreceptor protein expression in progressionof premalignant lesions to head and neck squamouscell carcinoma. Clin Cancer Res 4:13-20, 1998.
29
. Hirai T, Kuwahara M, Yoshida K, et al:Clinical results of transhiatal esophagectomyfor carcinoma of the lower thoracic esophagusaccording to biological markers. Dis Esophagus11:221-225, 1998.
30.
Mayer A, Takimoto M, Fritz E, et al:The prognostic significance of proliferating cellnuclear antigen, epidermal growth factor receptor,and mdr gene expression in colorectal cancer.Cancer 71:2454-2460, 1993.
31.
Tateishi M, Ishida T, Kohdono S, et al:Prognostic influence of the co-expression ofepidermal growth factor receptor and c-erbB-2protein in human lung adenocarcinoma. SurgOncol 3:109-113, 1994.
32.
Saeki T, Salomon DS, Johnson GR, etal: Association of epidermal growth factor-relatedpeptides and type i receptor tyrosine kinasereceptors with prognosis of humancolorectal carcinomas. Jpn J Clin Oncol25:240-249, 1995.
33.
Xia W, Lau YK, Zhang HZ, et al: Combinationof EGFR, HER-2/neu, and HER-3 isa stronger predictor for the outcome of oralsquamous cell carcinoma than any individualfamily members. Clin Cancer Res 5:4164-4174, 1999.
34.
Khorana AA; Ryan CK, Eberly S, et al:EGFR expression and survival in stage II, IIIand IV colon cancer (abstract 1272). Proc AmSoc Clin Oncol 22:317, 2003.
35.
Chen X, Yeung TK, Wang Z: Enhanceddrug resistance in cells coexpressing ErbB2with EGF receptor or ErbB3. Biochem BiophysRes Commun 277:757-763, 2000.
36.
Akimoto T, Hunter NR, Buchmiller L,et al: Inverse relationship between epidermalgrowth factor receptor expression andradiocurability of murine carcinomas. ClinCancer Res 5:2884-2890, 1999.
37.
Bruns CJ, Harbison MT, Davis DW, etal: Epidermal growth factor receptor blockadewith C225 plus gemcitabine results in regressionof human pancreatic carcinoma growingorthotopically in nude mice by antiangiogenicmechanisms. Clin Cancer Res 6:1936-1948,2000.
38.
Fan Z, Baselga J, Masui H, et al: Antitumoreffect of anti-epidermal growth factor receptormonoclonal antibodies plus cisdiamminedichloroplatinumon well establishedA431 cell xenografts. Cancer Res 53:4637-4642, 1993.
39.
Prewett MC, Hooper AT, Bassi R, et al:Enhanced antitumor activity of anti-epidermalgrowth factor receptor monoclonal antibodyIMC-C225 in combination with irinotecan(CPT-11) against human colorectal tumor xenografts.Clin Cancer Res 8:994-1003, 2002.
40.
Overholser JP, Prewett MC, Hooper AT,et al: Epidermal growth factor receptor blockadeby antibody IMC-C225 inhibits growth ofa human pancreatic carcinoma xenograft innude mice. Cancer. 89:74-82, 2000.
41.
Sirotnak FM, Zakowski MF, Miller VA,et al: Efficacy of cytotoxic agents against humantumor xenografts is markedly enhancedby coadministration of ZD1839 (Iressa), aninhibitor of EGFR tyrosine kinase. Clin CancerRes 6:4885-4892, 2000.
42.
Masui H, Kawamoto T, Sato JD, et al:Growth inhibition of human tumor cells inathymic mice by anti-epidermal growth factorreceptor monoclonal antibodies. Cancer Res44:1002-1007, 1984.
43.
Wu X, Fan Z, Masui H, et al: Apoptosisinduced by an anti-epidermal growth factorreceptor monoclonal antibody in a humancolorectal carcinoma cell line and its delay byinsulin. J Clin Invest 95:1897-1905, 1995.
44.
Moyer JD, Barbacci EG, Iwata KK, etal: Induction of apoptosis and cell cycle arrestby CP-358,774, an inhibitor of epidermalgrowth factor receptor tyrosine kinase. CancerRes 57:4838-4848, 1997.
45.
Perrotte P, Matsumoto T, Inoue K, et al:Anti-epidermal growth factor receptor antibodyC225 inhibits angiogenesis in human transitionalcell carcinoma growing orthotopicallyin nude mice. Clin Cancer Res 5:257-265,1999.
46.
Fan Z, Mendelsohn J, Masui H, et al:Regulation of epidermal growth factor receptorin NIH3T3/HER14 cells by antireceptor monoclonalantibodies. J Biol Chem 268:21073-21079, 1993.
47.
Kedar D, Baker CH, Killion JJ, et al:Blockade of the epidermal growth factor receptorsignaling inhibits angiogenesis leadingto regression of human renal cell carcinomagrowing orthotopically in nude mice. Clin CancerRes 8:3592-3600, 2002.
48.
Ciardiello F, Caputo R, Bianco R, et al:Inhibition of growth factor production and angiogenesisin human cancer cells by ZD1839(Iressa), a selective epidermal growth factorreceptor tyrosine kinase inhibitor. Clin CancerRes 7:1459-1465, 2001.
49.
Karashima T, Sweeney P, Slaton JW, etal: Inhibition of angiogenesis by theantiepidermal growth factor receptor antibodyImClone C225 in androgen-independent prostatecancer growing orthotopically in nudemice. Clin Cancer Res 8:1253-1264, 2002.
50.
Ennis BW, Lippman ME, Dickson RB:The EGF receptor system as a target for antitumortherapy. Cancer Invest 9:553-562,1991.
51.
Herbst RS, Shin DM: Monoclonal antibodiesto target epidermal growth factor receptor-positive tumors: A new paradigm forcancer therapy. Cancer. 94:1593-1611, 2002.
52.
Viloria-Petit A, Crombet T, Jothy S, etal: Acquired resistance to the antitumor effectof epidermal growth factor receptor-blockingantibodies in vivo: A role for altered tumorangiogenesis. Cancer Res 61:5090-5101,2001.
53.
Prenzel N, Zwick E, Daub H, et al: EGFreceptor transactivation by G-protein-coupledreceptors requires metalloproteinase cleavageof ProHB-EGF. Nature 402:884-888.
54.
Cox G, Jones JL, O’Byrne KJ: Matrixmetalloproteinase 9 and the epidermal growthfactor signal pathway in operable non-small celllung cancer. Clin Cancer Res 6:2349-2355,2000.
55.
Karnes WE Jr, Weller SG, Adjei PN, etal: Inhibition of epidermal growth factor receptorkinase induces protease-dependentapoptosis in human colon cancer cells. Gastroenterology.114:930-939, 1998.
56.
Huang SM, Bock JM, Harari PM: Epidermalgrowth factor receptor blockade withC225 modulates proliferation, apoptosis, andradiosensitivity in squamous cell carcinomasof the head and neck. Cancer Res 59:1935-1940, 1999.
57.
Baselga J, Pfister D, Cooper MR, et al:Phase I studies of anti-epidermal growth factorreceptor chimeric antibody C225 alone andin combination with cisplatin. J Clin Oncol18:904-914, 2000.
58.
Saltz L, Rubin M, Hochster H, et al:Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectalcancer (CRC) that expresses epidermal growthfactor receptor (EGFR) (abstract 7). Proc AmSoc Clin Oncol 20:3a, 2001.
59.
Saltz L, Meropol NJ, Loehrer PJ, et al:Single agent IMC-C225 (Erbituxâ¢) has activityin CPT-11-refractory colorectal cancer(CRC) that expresses the epidermal growth factorreceptor (EGFR) (abstract 504). Proc AmSoc Clin Oncol 21:127a, 2002.
60.
Cunningham D, Humblet Y, Siena S, etal: Cetuximab (C225) alone or in combinationwith irinotecan (CPT-11) in patients with epidermalgrowth factor receptor (EGFR)-positive,irinotecan-refractory metastatic colorectal cancer(MCRC) (abstract 1012). Proc Am Soc ClinOncol 22:252, 2003.
61.
Schoffski P, Lutz M, Folprecht G, et al:Cetuximab (C225) plus irinotecan (CPT-11)plus infusional 5FU-folinic acid (FA) is safeand active in metastatic colorectal cancer(MCRC), that expresses epidermal growth factorreceptor (EGFR) (abstract 633). Proc AmSoc Clin Oncol 21:159a, 2002.
62.
Rosenberg AH, Loehrer PJ, Needle MN,et al: Erbitux (IMC-C225) plus weeklyirinotecan (CPT-11), fluorouracil (5FU) andleucovorin (LV) in colorectal cancer (CRC) thatexpresses the epidermal growth factor receptor(EGFr) (abstract 536). Proc Am Soc ClinOncol 21:135a, 2002.
63.
Van Laethem J-L, Raoul J-L, Mitry E, etal: Cetuximab (C225) in combination with biweeklyirinotecan (CPT-11), infusional 5-fluorouracil(5-FU) and folinic acid (FA) in patients(pts) with metastatic colorectal cancer (CRC)expressing the epidermal growth factor receptor(EGFR). Preliminary safety and efficacyresults (abstract 1058). Proc Am Soc Clin Oncol22:264a, 2003.
64.
Yang XD, Jia XC, Corvalan JR, et al:Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancertherapy. Crit Rev Oncol Hematol 38:17-23,2001.
65.
Yang XD, Jia XC, Corvalan JR, et al:Eradication of established tumors by a fullyhuman monoclonal antibody to the epidermalgrowth factor receptor without concomitantchemotherapy. Cancer Res 59:1236-1243,1999.
66.
Figlin RA, Belldegrun AS, Crawford J,et al: ABX-EGF, a fully human anti-epidermalgrowth factor receptor (EGFR) monoclonalantibody (mAb) in patients with advanced cancer:Phase 1 clinical results (abstract 35). ProcAm Soc Clin Oncol 21:10a, 2002.
67.
Roskos L, Lohner M, Osborn R, et al:Low pharmacokinetic variability facilitatesoptimal dosing of ABX-EGF in cancer patients(abstract 362). Proc Am Soc Clin Oncol 21:91a,2002.
68.
Meropol NJ, Berlin J, Hecht JR, et al:Multicenter study of ABX-EGF monotherapyin patients with metastatic colorectal cancer(abstract 1026). Proc Am Soc Clin Oncol22:256, 2003.
69.
Burger A, Heiss NS, Kreysch HG, et al:The humanized monoclonal anti-EGFR antibodyEMD72000 potently inhibits the growthof EGFR-expressing human tumor xenograftsinsensitive to chemotherapeutic drugs (abstract5719). Proc Am Assoc Cancer Res 44:1139,2003.
70.
Tewes M, Schleucher N, Dirsch O, et al:Results of a phase I trial of the humanizedanti epidermal growth factor receptor (EGFR)monoclonal antibody EMD 72000 in patientswith EGFR expressing solid tumors (abstract378). Proc Am Soc Clin Oncol 21:95a,2002.
71.
Tabernero J, Rojo F, Jimenez E, et al: Aphase I PK and serial tumor and skin pharmacodynamic(PD) study of weekly (q1w), every2-week (q2w) or every 3-week (q3w) 1-hour(h) infusion EMD72000, a humanized monoclonalanti-epidermal growth factor receptor(EGFR) antibody, in patients (pt) with advanced tumors (abstract 770). Proc Am Soc ClinOncol 22:192, 2003.
72.
Kris MG, Natale RB, Herbst RS: A phaseII trial of ZD1839 (‘Iressa’) in advanced nonsmallcell lung cancer (NSCLC) patients whohad failed platinum- and docetaxel-based regimens(IDEAL 2) (abstract 1166). Proc Am SocClin Oncol 21:292a, 2002.
73.
Fukuoka M, Yano S, Giaccone G: Finalresults from a phase II trial of ZD1839 (‘Iressa’)for patients with advanced non-small-cell lungcancer (IDEAL 1) (abstract 1188). Proc Am SocClin Oncol 21:298a, 2002.
74.
Goss GD, Stewart DJ, Hirte H: Initialresults of part 2 of a phase I/II pharmacokinetics(PK), pharmacodynamic (PD) and biologicalactivity study of ZD1839 (Iressa): NCICCTG IND.122 (abstract 59). Proc Am Soc ClinOncol 21:16a, 2002.
75.
Dorligschaw O, Kegel T, Jordan K, etal: ZD 1839 (Iressa)-based treatment as lastlinetherapy in patients with advancedcolorectal cancer (ACRC) (abstract 1494). ProcAm Soc Clin Oncol 22:272, 2003.
76.
Braun AH, Dirsch O, Hilger RA, et al:Preclinical evaluation of the combination ofepidermal growth factor inhibitor ZD1839(Iressa) and irinotecan (SN-38) in human coloncancer cells (abstract 329). Proc Am SocClin Oncol 21:83a, 2002.
77.
Cho CD, Fisher GA, Halsey J, et al: Aphase II study of gefitinib in combination withFOLFOX-4 (IFOX) in patients withunresectable or metastatic colorectal cancer(abstract 1062). Proc Am Soc Clin Oncol22:265, 2003.
78.
Kim TE, Murren JR: Erlotinib OSI/Roche/Genentech. Curr Opin Investig Drugs3:1385-1395, 2002.
79.
Perez-Soler R, Chachoua A, HubermanM, et al: Phase II trial of the epidermal growthfactor receptor (EGFR) tyrosine kinase inhibitorOSI-774, following platinum-based chemotherapy,in patients (pts) with advanced, EGFRexpressing,non-small cell lung cancer(NSCLC) (abstract 1235). Proc Am Soc ClinOncol 20:310a, 2001.
80.
Senzer NN, Soulieres D, Siu L, et al:Phase 2 evaluation of OSI-774, a potent oralantagonist of the EGFR-TK in patients withadvanced squamous cell carcinoma of the headand neck (abstract 6). Proc Am Soc Clin Oncol20:2a, 2001.
81.
Finkler N, Gordon A, Crozier M, et al:Phase 2 evaluation of OSI-774, a potent oralantagonist of the EGFR-TK in patients withadvanced ovarian carcinoma (abstract 831).Proc Am Soc Clin Oncol 20:208a, 2001.
82.
Kerfoot CA, Thompson J, RichardsonM, et al: In vitro activity of Tarceva in primarylung and colon cancer cells (abstract R3796).Proc Am Assoc Cancer Res 44:755, 2003.
83.
Townsley C, Major P, Siu LL, et al: PhaseII study of OSI-774 in patients with metastaticcolorectal cancer (abstract 179). Eur J Cancer38(suppl 7):57, 2002.
84.
Akita RW, Sliwkowski MX: Preclinicalstudies with erlotinib (Tarceva). Semin Oncol30(3 suppl 7):15-24, 2003.
85.
Malik SN, Siu LL, Rowinsky EK, et al:Pharmacodynamic evaluation of the epidermalgrowth factor receptor inhibitor OSI-774 inhuman epidermis of cancer patients. Clin CancerRes 9:2478-2486, 2003.
86.
Baselga J, Rischin D, Ranson M, et al:Phase I safety, pharmacokinetic, and pharmacodynamictrial of ZD1839, a selective oralepidermal growth factor receptor tyrosine kinaseinhibitor, in patients with five selectedsolid tumor types. J Clin Oncol 20:4292-4302,2002.
87.
Albanell J, Rojo F, Averbuch S, et al:Pharmacodynamic studies of the epidermalgrowth factor receptor inhibitor ZD1839 in skinfrom cancer patients: Histopathologic andmolecular consequences of receptor inhibition.J Clin Oncol 20:110-124, 2002.
88.
Liu W, Innocenti F, Chen P, et al: Interethnicdifference in the allelic distribution ofhuman epidermal growth factor receptor intron1 polymorphism. Clinical Cancer Research.2003;9(3):1009-12.
89.
Gebhardt F, Zanker KS, Brandt B: Modulationof epidermal growth factor receptor genetranscription by a polymorphic dinucleotiderepeat in intron 1. J Biol Chem 274:13176-13180, 1999.
90.
Moriai T, Kobrin MS, Hope C, et al:Variant epidermal growth factor receptor exhibitsaltered type alpha transforming growthfactor binding and transmembrane signaling.Proc Natl Acad Sci U S A 91:10217-10221,1994.
91.
Cohen SJ, Alpaugh RK, Allard J, et al:Isolation and characterization of circulatingtumor cells in patients with colorectal cancer(abstract 1186). Proc Am Soc Clin Oncol22:295, 2003.