Evolving Chemoradiation Treatment Strategies for Locally Advanced Non-Small-Cell Lung Cancer
Survival for patients with stage III nonSMQ-8211-SMQsmall-cell lung cancer hasgradually improved in recent years, with median survival times increasingfrom less than 10 months to more than 18 months. These increasesare thought to result primarily from advances in chemoradiation. Thisarticle reviews major advances in the development of chemoradiationfor patients with locally advanced nonSMQ-8211-SMQsmall-cell lung cancer. Resultsfrom cooperative group trials suggest that concurrent chemoradiationis superior to sequential therapy and may replace sequential therapy asthe new standard of care in patients with good performance status.Technological advances such as 18F-fluorodeoxyglucose positron emissiontomography (PET) staging can be used to improve patient selectionand predict survival. Locoregional control may be improved byaltering radiation fractionation or delivery (eg, hyperfractionation, highdoseinvolved-volume radiotherapy, 3D conformal radiotherapy). Novelagents and regimens in combination with radiation are being investigatedto further improve therapeutic outcomes.
ABSTRACT: Survival for patients with stage III non-small-cell lung cancer has gradually improved in recent years, with median survival times increasing from less than 10 months to more than 18 months. These increases are thought to result primarily from advances in chemoradiation. This article reviews major advances in the development of chemoradiation for patients with locally advanced nonSMQ-8211-SMQsmall-cell lung cancer. Results from cooperative group trials suggest that concurrent chemoradiation is superior to sequential therapy and may replace sequential therapy as the new standard of care in patients with good performance status. Technological advances such as 18F-fluorodeoxyglucose positron emission tomography (PET) staging can be used to improve patient selection and predict survival. Locoregional control may be improved by altering radiation fractionation or delivery (eg, hyperfractionation, highdose involved-volume radiotherapy, 3D conformal radiotherapy). Novel agents and regimens in combination with radiation are being investigated to further improve therapeutic outcomes.
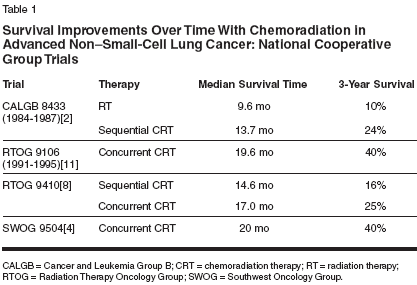
Chemoradiation therapy for patients with locally advanced (stage IIIA/IIIB) non-smallcell lung cancer has improved considerably in recent years. Because of the advanced tumor stage and/or unresectability of these tumors, curative surgery is not an option.[1] In such cases, radiation therapy and chemotherapy are the standard of care. A review of efficacy data from national cooperative group trials enrolling non- small-cell lung cancer patients with similar eligibility requirements shows significant improvements in survival: the overall median survival time for non-small-cell lung cancer patients was less than 10 months in trials starting in 1984[2] compared to more than 18 months (and in one case, more than 24 months[4]) in trials initiated in the 1990s (Table 1).[2-5] Improvements have also been observed in 3-year survival rates. The reason for such improvements cannot be explained by stage migration alone. This review will discuss the evolution of these changes, and how chemoradiation has improved and continues to improve patient outcomes for those with non-small-cell lung cancer. The first large chemoradiation trial to show a survival benefit was Cancer and Leukemia Group B (CALGB) 8433.[2] This trial demonstrated a significant survival advantage for patients with unresectable stage III non-smallcell lung cancer who received sequential chemotherapy and radiation compared with radiation alone. Patients were randomly assigned to receive induction chemotherapy (cisplatin/ vinblastine) before radiation therapy (6,000 cGy in 30 fractions beginning on day 50) or radiation therapy alone (6,000 cGy beginning on day 1 for 6 to 7 weeks). Significant improvements with the combined-modality regimen prompted the study to close early after enrolling only 155 patients. Radiographically, the tumor response rate was 56% for patients receiving the combined modality compared with 43% for patients receiving radiation therapy alone (P = .092). Median survival times were 13.7 months for the chemoradiation therapy group and 9.6 months for the radiation-only group (P = .012); the 5-year survival rates were 17% and 6%, respectively, indicating 2.8-fold increase. Survival Benefit With Concurrent Chemoradiation The survival benefit of combined chemotherapy and radiation therapy for patients with advanced non-smallcell lung cancer has been supported by several additional phase III trials,[ 2,5-8] including another large trial (Radiation Therapy Oncology Group [RTOG] 9410)[8] that evaluated the efficacy of sequential vs concurrent chemoradiation protocols. RTOG 9410 enrolled 611 patients over 4 years (1994 to 1998) with a minimum follow-up of 4 years (Table 2).[8,9] The study design compared the CALGB sequential regimen of chemotherapy (cisplatin/vinblastine) before radiation therapy (60 Gy for 7 weeks at day 50) with hyperfractionated radiation (69.9 Gy twice daily for 6 weeks) or standard radiation therapy (60 Gy daily for 7 weeks) administered concurrently with chemotherapy. The pilot data for the two concurrent arms came from two RTOG phase II trials. In the first trial, patients were treated with vinblastine/cisplatin given concurrently with hyperfractionated radiation therapy, and the resulting overall survival was 12.2 months.[10] In the second RTOG trial, oral etoposide and cisplatin were given concurrently with hyperfractionated radiation therapy, and the resulting median survival was 20 months.[11]
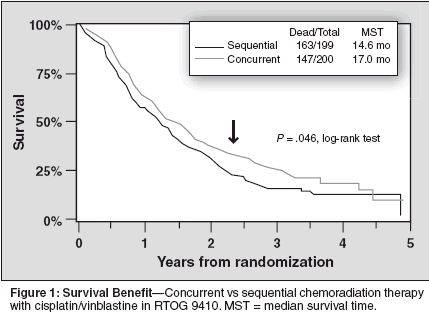
At a median potential follow-up time of 40 months, the RTOG 9410 trial demonstrated preliminary median survival times of 14.6 months with sequential therapy, 17.0 months with concurrent therapy with daily radiation therapy, and 15.2 months with hyperfractionated concurrent therapy.[8] Overall, significant improvements in survival (P = .046) were observed with concurrent chemotherapy and radiation therapy (Figure 1). However, reversible grade 3/4 nonhematologic toxicities were substantially higher with concurrent compared with sequential therapies, especially for esophagitis.[8] Interestingly, while esophagitis was still considered to be relatively low, the rate of pneumonitis (regardless of when radiation therapy was administered) was substantial and may be an underappreciated toxicity with such protocols. Indeed, most of the late grade 5 toxicities in the study, which occurred at a rate of only 2%, were actually pneumonitis related. With this in mind, a quality-adjusted survival analysis that subtracts the time spent with toxicity and/or relapse from survival was performed to determine if the improvement in survival outcome data outweighed the increase in reversible nonhematologic toxicities, as seen with concurrent therapy.[12]
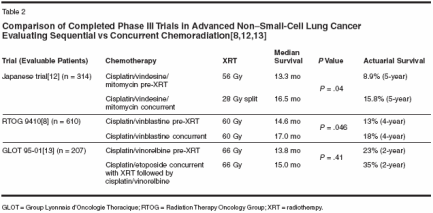
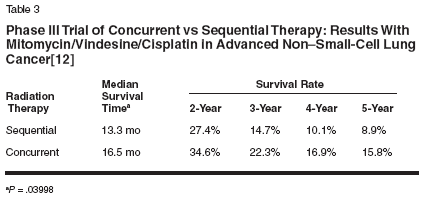
Treatments were compared over the entire spectrum of toxicity and relapse values for 593 evaluable patients. Despite the increase in reversible nonhematologic toxicities observed with concurrent chemoradiation, the overall mean toxicity was actually highest in the sequential arm, where treatment time was longest. Of the concurrent therapies, concurrent chemoradiation with once-daily radiation therapy resulted in the optimal quality-adjusted survival and supported the use of concurrent chemoradiation as a preferred treatment for patients with advanced non- small-cell lung cancer. A second phase III study comparing concurrent vs sequential therapy has shown similar results.[13] In this Japanese study, 320 patients with unresectable stage III non-small-cell lung cancer were randomized to receive either concurrent radiation (28 Gy at 2 Gy per fraction, and 5 fractions per week for a total of 14 fractions beginning on day 2) with chemotherapy (cisplatin at 80 mg/m2 on days 1 and 29; vindesine at 3 mg/m2 on days 1, 8, 29, and 36; and mitomycin (Mutamycin at 8 mg/m2 on days 1 and 29) or chemotherapy followed by sequential radiation (56 Gy, or 2 Gy per fraction, and 5 fractions per week for a total of 28 fractions). Overall, the response rate for the concurrent arm was 84% compared with 66% for the sequential arm, showing a significant improvement in response with concurrent therapies (P = .0002). The median survival time was 16.5 months with concurrent therapy vs 13.3 months with sequential therapy (P = .03998; Tables 2 and 3). While myelosuppression was found to be significantly greater in patients receiving concurrent chemoradiation therapy, the increased response rate showed concurrent therapy to provide a benefit in long-term survival with acceptable toxicity.
The Groupe Lyonnais d'Oncologie Thoracique (GLOT) trial also evaluated the survival effects of concurrent chemoradiation therapy as compared with sequential therapy in patients with unresectable, locally advanced stage III non-small-cell lung cancer.[ 14] This study included 212 patients randomized to receive cisplatin/ vinorelbine (Navelbine) followed by thoracic radiation (66 Gy in 33 fractions for 6.5 weeks), or concurrent radiation therapy started on day 1 with two concurrent cycles of cisplatin/ etoposide followed by cisplatin/ vinorelbine (Table 2). The total dose of cisplatin was equivalent in both groups. The preliminary report of this study demonstrated a trend in favor of concurrent therapy; however, these differences were not statistically significant. Based on the preliminary results of RTOG 9410 and the other supportive phase III studies, concurrent chemoradiation therapy appears to offer a survival benefit over sequential therapy. While sequential therapy has been associated with lower toxicity, a reduction in distant failures, and fewer treatment interruptions, concurrent therapy may offer several additional advantages such as a synergism between the two modalities and a reduction in chest failures (ie, regional tumor failures). It is likely that concurrent therapy may replace sequential therapy as the new standard, at least in investigational therapy, and probably in nonprotocol therapy for patients with good performance status. Significant benefits with concurrent therapy have also been observed in studies of other solid tumors, including head and neck carcinomas (RTOG 9111 and RTOG 9003).[15,16] In these solid tumors, the addition of chemotherapy to radiation results in an increase in cure rate by improving tumor control in the region and eliminating or delaying the emergence of metastatic disease. The efficacy and toxicity associated with concurrent therapy are likely to be optimized based on dose, schedule, and type of chemotherapeutic agent. Similarly, the quality and mode of radiation delivery could be optimized by taking advantage of technical improvements in patient selection and staging. Such improvements might include imaging techniques, radiotherapy sequencing and fractionation, image guidance during radiation therapy, brachytherapy (high radiation doses provided directly to a tumor through implantation of small radioactive seeds), radiation intensity modulation, and radiation dose escalation. Improving Tumor Definition One of the more important factors for improving patient survival may be the use of techniques that aid in tumor definition. Typically, tumors or target lesions are defined using three-dimensional (3D) treatment planning computed tomography (CT) and diagnostic CT scans. However, positron emission tomography (PET) appears to be an effective means of detecting and staging mediastinal metastases in patients with non-small-cell lung cancer because of its higher sensitivity.[ 17] PET scans are able to locate target hot zones by excluding atelectasis and uninvolved lymph node stations, and thus may achieve greater local control. Recent studies have indicated that the use of 18F-fluorodeoxyglucose (FDG)-PET staging can serve as a powerful tool to improve patient selection[18] and effectively predict patient survival.[19] FDG-PET staging also appears to be superior to CT for staging of non- small-cell lung cancer.[20] In addition, the residual metabolic rate of glucose as measured by FDG-PET at the primary lesion appears to be an effective surrogate marker of tumor response to induction therapy with combined modality.[21] Techniques such as CT and PET scans may also improve treatment outcomes for newer modalities such as high-dose involved-volume radiotherapy, which is currently under investigation. Interestingly, a recent retrospective analysis of clinical course and tumor volume data from 135 patients with non-small-cell lung cancer revealed that long-term local control and survival may depend on the tumor volume and total dose of radiation. The study found that while conventional doses (≥ 70 Gy) may be effective in local control of small tumors (< 100 cm3, maximum diameter of 6 cm), these doses were unlikely to control tumors ≥ 100 cm3 and supported the use of dose escalation in patients with non-small-cell lung cancer.[22] Radiation Therapy Advances That Impact Survival Concurrent therapy studies clearly demonstrate that the quality of radiation therapy can impact tumor control and survival. Nonetheless, the timing of chemoradiation has yet to be optimized. One study, the Locally Advanced Multimodality Protocol (LAMP) ACR 427, has been designed to evaluate the long-term survival benefits of induction chemotherapy prior to or following concurrent chemoradiation as compared with sequential chemoradiation.[23] The trial includes patients with a good performance status and unresectable stage III non-small-cell lung cancer who are randomized to one of three treatment arms: (1) two cycles of paclitaxel at 200 mg/m2 with carboplatin (Paraplatin) at an area under the curve (AUC) of 6 followed by daily radiation to 63.0 Gy; (2) weekly paclitaxel at 45 mg/m2 with carboplatin at an AUC of 2 concurrent with daily radiation to 63.0 Gy; and (3) radiation beginning on day 1 (to 63.0 Gy) with concurrent paclitaxel 45 mg/m2 and carboplatin at AUC 2 for 7 weeks, followed by two cycles of paclitaxel at 200 mg/m2 and carboplatin at AUC 6. All arms were to be compared to a historical sequential chemoradiation regimen (RTOG 8808), which observed a median survival time of 13.7 months. Preliminary analysis of phase II data showed that the most promising therapy was chemoradiation followed by chemotherapy (arm 3), and the least promising was chemotherapy followed by chemoradiation (arm 2).[23,24] Patient accrual in arm 2 was terminated after evaluating data for the first 80 patients enrolled because early survival analysis did not demonstrate a survival benefit as compared with the historical sequential chemoradiation data. After a median follow-up time of 26 months, median survival time was 16.1 months for arm 3, 12.5 months for arm 1, and 11.0 months for arm 2, suggesting adjuvant chemotherapy following chemoradiation to be the arm most worthy of further study.[24] Thus, although the optimal radiation dose and fields remain unknown in the setting of chemotherapy, combinedmodality chemoradiation protocols continue to demonstrate improvements in patient survival. Other attempts to enhance patient survival include strategies to improve locoregional control of radiation by increasing radiation dose intensity through the administration of multiple and/or altered radiation fractions each day. Methods of altering radiation dose intensity include altering fractionation (eg, hyperfractionated accelerated radiotherapy) or increasing lo- cal control in the initially involved area by delivery of high-dose radiation (high-dose involved-volume radiotherapy and 3D conformal radiotherapy [CRT]). The effects of accelerated hyperfractionation radiation therapy in non-small-cell lung cancer have recently been evaluated in several trials.[ 10,25-28] The Continuous Hyperfractionated Accelerated Radiotherapy (CHART) trial compared hyperfractionated radiation (36 fractions of 1.5 Gy/fraction given as 3 fractions per day for 12 days, for a total dose of 54 Gy) with standard radiotherapy (60 Gy over 6 weeks).[25] The hyperfractionated dose was found to significantly improve 2-year survival by 9% compared to standard radiotherapy, and improved survival was directly linked to a decrease in local tumor progression. A follow-up quality- of-life and toxicity analysis showed that the hyperfractionated dose did not increase short- or long-term morbidity as compared with the standard therapy.[26] The Hyperfractionated Accelerated Radiation Therapy (HART) trial, sponsored by the Eastern Cooperative Oncology Group (ECOG 4593), evaluated 1.5- to 1.8- Gy fractions given three times daily for 16 days (total dose of 57.6 Gy).[27] The median survival for patients enrolled on this trial was 13 months, with a 1-year survival rate of 57% A dose-escalation study by Saunders and colleagues of continuous hyperfractionated accelerated radiotherapy weekend-less (CHARTWEL) combined with neoadjuvant chemotherapy also showed a survival benefit for the combination compared to radiation therapy alone.[28] Locoregional control at 2 years was 37% for patients receiving CHARTWEL at 54 Gy, 55% for those receiving CHARTWEL at 60 Gy, and 72% for patients receiving CHARTWEL at 60 Gy with neoadjuvant chemotherapy. Unfortunately, there remains a development of recurrence in 80% of patients treated with such therapies, and 60% will develop distant metastases. Interestingly, hyperfractionated radiation studies performed in the United States with concurrent chemotherapy have not shown any apparent benefit. Because of the high rate of distant metastases and local recurrence, intensified radiotherapy techniques are now being integrated with 3D-CRT techniques, a technique based on virtual simulation that reconstructs the tumor and surrounding organs in 3D and creates custom-shaped radiation fields around the tumor site in order to avoid excessive exposure to nontarget tissues. Three-dimensional CRT is a high-precision technique based on the 3D volumetric definition of the tumor and the anatomy of critical organs. After obtaining a series of images of the transverse section of the treated region, a digital reconstruction of these volumes is produced. A simulation program is then used to determine the optimal orientation of the irradiation beam and the number of fields, which allows the calculation of the dose to be conducted on the whole treated volume and not just the surface (as is done for conventional radiotherapy).[ 29] By applying an intensity modulation technique to 3D-CRT, the dose distribution can be changed dynamically, permitting an irregular dose distribution that conforms exactly to the volume of the target. Belderbos and colleagues are conducting a phase I/II dose-escalation trial using 3D-CRT to evaluate the maximum tolerated dose in patients with stage I-IIIB non-small-cell lung cancer.[30] Patients who had previously received elective nodal irradiation or who had received chemotherapy 6 weeks prior to the study were excluded. Fifty-five patients were divided into five risk groups (defined according to mean lung dose), and each group was treated 5 days a week with 2.25 Gy per fraction for 6 weeks. When fewer than 30 fractions were prescribed, two fractions were administered per day. Within each group, the dose was escalated with three fractions per step (6.75 Gy). An interim analysis revealed that 17 patients received a dose of 74.3 Gy and 23 received a dose of 81.0 Gy. Radiation pneumonitis occurred in seven patients (grade 4, n = 1), while esophageal toxicity was mild. Fifty patients were evaluable for response at 3 months: 6 patients experienced a com- plete response, 38 had a partial response, 5 patients had stable disease, and 1 patient experienced progressive disease. To date, the radiation dose has been safely escalated to 87.8 Gy, and the maximum tolerated dose has not yet been reached. Phase II/III studies of 3D-CRT are currently ongoing. Xu et al recently conducted an efficacy study of 3D-CRT vs conventional radiotherapy in 135 non-smallcell lung cancer patients.[31] The 62 patients enrolled on the 3D-CRT arm received 48 to 64 Gy in 6 to 8 fractions over 2 to 3 weeks (6-8 Gy per fraction). The remaining 73 patients received conventional radiotherapy at a total dose of 60 to 70 Gy in 30 to 35 fractions over 6 to 7 weeks. After 3 months, 45% of patients receiving conventional radiotherapy experienced a complete remission of lesions compared with 77.8% of patients on the 3D-CRT arm. The 1- and 2-year survival rates significantly favored the 3D-CRT treatment (42.5% vs 77.8% and 30.1% vs. 48.6%, respectively). There were no significant differences in the incidence of radiation-induced lung and esophageal injuries, suggesting that the survival benefit of 3D-CRT may be a preferred treatment protocol for patients with non-small-cell lung cancer. Singh et al recently performed a retrospective review of 207 patients treated with 3D-CRT.[32] Patients received either sequential chemotherapy, concurrent chemotherapy, or radiation alone with a median prescription dose of 70 Gy delivered in once-daily 2-Gy fractions. Of the 207 patients enrolled, 16 (8%) developed acute or late grade 3-5 esophageal toxicity, and 1 patient died due to grade 5 esophageal toxicity. Concurrent chemotherapy was significantly associated with a risk of grade 3-5 esophageal toxicity. However, no such toxicities were observed when the maximal point dose to the esophagus was less than 58 Gy (P = .0001). The number of patients who developed grade 3-5 esophageal toxicity in the absence of chemotherapy was too small to statistically evaluate for a relationship between maximal dose and esophagitis. The authors concluded that there may be a maximal radiation point dose thresh old of 58 Gy for the esophagus in patients with non-small-cell lung cancer receiving concurrent chemotherapy with 3D-CRT.
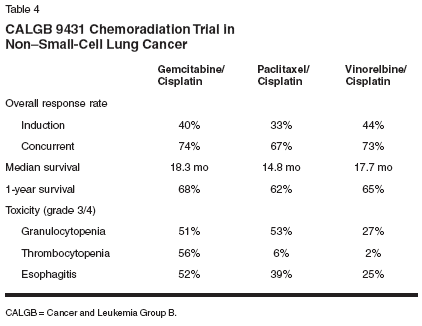
These results are similar to a study performed at the Memorial Sloan- Kettering Cancer Center, which observed a survival advantage with the use of induction chemotherapy prior to 3D-CRT compared to 3D-CRT alone.[33] Of the 152 patients enrolled, 70 received radiation alone (median dose: 70.2 Gy) and 82 received chemotherapy (generally a platinum-based regimen) prior to radiation (mean dose: 64.8 Gy). The median survival time for patients receiving radiation alone was 11.7 months compared with 18.1 months for those receiving combined-modality therapy. A reduction in grade 3 or higher nonhematologic toxicities was also noted in the combined-treatment group (16% vs 20%), suggesting that chemotherapy provides additional benefit to those receiving radiation therapy alone. Currently, standard-dose extendedvolume radiotherapy is the radiotherapy of choice in many RTOG trials and has become the standard in many clinics across the United States. With proper patient selection and within settings of cooperative groups such as the RTOG, radiation doses of 90 to 100 cGy have been administered to selected patients with smaller volumes employing involved-volume radiotherapy.[34] Adjuvant Chemotherapy and Novel Agents With Radiation As with postoperative chemotherapy for early-stage non-small-cell lung cancer, the role of adjuvant systemic therapy in the setting of radiation treatment remains unclear. Early phase I/II results to determine the maximum tolerated dose of daily paclitaxel in combination with radiotherapy (to 60 Gy) concurrent with or following carboplatin administration for 1 to 4 cycles have shown the combination of paclitaxel (up to 11 mg/m2/d) with radiation to be well tolerated.[35] Novel approaches such as longterm administration of less toxic drugs, use of newer agents, and targeted biologic therapies show promise as alternatives or additions to the current therapeutic armamentarium. For example, the use of several novel antitumor agents are currently under evaluation for the treatment of tumors in conjunction with radiation therapy. Systemic agents that are currently be- ing studied in phase I/II trials include cyclooxygenase-2 inhibitors (RTOG trial C-0128); SU-5416, a vascular endothelial growth factor receptor tyrosine kinase inhibitor (RTOG trial S- 1021); angiostatin, an angiogenesis inhibitor (university-sponsored trial); and R11577, a farnesyltransferase inhibitor (RTOG trial 0020). In the past few years, advances have been made in the identification of markers that may act as prognostic indicators of cancer cell progression. Epidermal growth factor receptor (EGFR) expression is one such marker that has shown promise as a potential marker of disease progression. The Southwest Oncology Group (SWOG) is conducting an Intergroup trial (S0023) with the National Cancer Institute of Canada and the North Central Cancer Treatment Group to evaluate chemoradiotherapy with or without the EGFR tyrosine kinase inhibitor gefitinib (Iressa) following chemoradiation in patients with non- small-cell lung cancer.[36] Phase II trials are planning to use an alternative design, ie, chemo-radiation with concurrent gefitinib or the anti-EGFR monoclonal antibody C225. New Systemic Therapies to Optimize Treatment The use of radiosensitizing agents to optimize treatment with radiation appears to be a likely method of improving patient survival. One randomized phase II study, CALGB 9431, examined several novel doublets with radiation therapy.[37] For all arms, cisplatin (80 mg/m2 on days 1, 22, 43, and 64) was administered for 2 cycles before radiation and again during radiation in combination with 1 of 3 newer chemotherapeutic agents: gemcitabine (Gemzar) (1,250 mg/m2 on days 1, 8, 22, and 29; 600 mg/m2 on days 43, 50, 64, and 71), paclitaxel (225 mg/m2 on days 1 and 22; 135 mg/ m2 on days 43 and 64), or vinorelbine (25 mg/m2 on days 1, 8, 15, 22, and 29; 15 mg/m2 on days 43, 50, 64, and 71). Higher doses were administered as induction therapy, and reduced doses were administered during concurrent therapy. The overall response rates follow- ing induction therapy and concurrent chemoradiotherapy were 40% and 74% for patients treated on the gemcitabine/cisplatin arm, 33% and 67% for patients receiving paclitaxel/ cisplatin, and 44% and 73% for the vinorelbine/cisplatin arm, respectively (Table 4). Grade 3/4 thrombocytopenia was significantly higher on the gemcitabine/cisplatin arm (56%) compared with the paclitaxel/cisplatin and vinorelbine/cisplatin arms (6% and 2%, respectively). Similarly, grade 3/4 esophagitis was greatest on the gemcitabine/cisplatin arm (52%). The results of this study suggest that doublet combinations may be administered with concurrent radiation with predictable toxicity. To improve therapeutic outcomes, the interaction between the radiosensitizer and the radiation dose must be optimized. A phase I trial has investigated the maximum tolerated dose of the radiosensitizing agent gemcitabine in patients with non-small-cell lung cancer who are undergoing radiation.[ 38] In this trial, dose-limiting toxicities occurred when gemcitabine at 125 mg/m2 was administered in combination with conventional radiotherapy. However, when combined with 3D-CRT, the maximum tolerated dose of gemcitabine was 190 mg/m2. Thus, optimization of the radiation therapy protocol with reduction in the radiation volume during 3D-CRT may allow substantially higher doses of an agent such as gemcitabine to be delivered effectively. The RTOG L-0017A trial is currently enrolling non-small-cell lung cancer patients to evaluate radiation combined with either carboplatin/ gemcitabine or gemcitabine/paclitaxel. Patients enrolled in this trial will receive escalating doses of gemcitabine in combination with a constant dose of carboplatin or escalating doses of paclitaxel. Patients on the gemcitabine/paclitaxel arm will receive dose escalation of only one drug at a time. In both arms, chemotherapy is administered with concurrent radiation therapy on the first day of chemotherapy. Patients on both arms can receive consolidation chemotherapy with gemcitabine/ carboplatin. New Standards of Care Studies now clearly demonstrate that chemoradiation provides a survival benefit over radiation therapy alone. This has resulted in the adoption of concurrent rather than sequential therapy. Nevertheless, the most effective chemotherapeutic protocol has yet to be determined. Whether a plateau in median survival has been reached with these combined therapies also remains unknown. Further improvements in patient outcomes will likely require a combination of approaches including optimizing patient selection and combined treatment dosing. Testing newer and novel systemic agents should also help to improve our understanding of the pathogenesis and treatment of non-small-cell lung cancer. Results of several ongoing phase III radiotherapy studies should help us to better understand and improve the quality of life in patients with advanced lung tumors. These studies include an RTOG trial that is evaluating amifostine (Ethyol) as a cytoprotectant to be given with two cycles of chemotherapy before a concurrent chemoradiation regimen; a CALGB study evaluating the effect of two cycles of induction therapy prior to a concurrent chemoradiation regimen; an Intergroup trial evaluating the role of surgery in patients with mediastinoscopy or mediastinotomy positive N2 disease; an ECOG trial studying the role of thalidomide (Thalomid); and a SWOG study of the impact of gefitinib following chemoradiation.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Gordon GS, Vokes EE: Chemoradiation for locally advanced, unresectable NSCLC. New standard of care, emerging strategies. Oncology 13:1075-1094, 1999.
2. Dillman RO, Herndon J, Seagren SL, et al: Improved survival in stage III non-smallcell lung cancer: Seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 88:1210-1215, 1996.
3. Sause W, Kolesar P, Taylor S IV, et al: Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer. Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 117:358-364, 2000.
4. Gandara D, Lovato L, Albain K, et al: Prolonged survival in pathologic stage IIIB non-small cell lung cancer with concurrent chemoradiotherapy followed by consolidation docetaxel: A phase II study (S9504) of the Southwest Oncology Group (SWOG) (abstract 1916). Proc Am Soc Clin Oncol 19:490a, 2000.
5. LeChevalier T, Arriagada R, Quoix E, et al: Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: First analysis of a randomized trial in 353 patients. J Natl Cancer Inst 83:417-423, 1991.
6. Schaake-Koning C, van den Bogaert W, Dalesio O, et al: Effects of concomitant cisplatin and radiotherapy on inoperable nonsmall- cell lung cancer. N Engl J Med 326:524- 530, 1992
7Marino P, Preatoni A, Cantoni A, et al: Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer: A meta-analysis. Cancer 76:593-601, 1995.
8. Curran W Jr, Scott C, Langer C, et al: Phase III comparison of sequential vs concurrent chemoradiation for patients (pts) with unresected stage III non-small cell lung cancer (NSCLC): Initial report of Radiation Therapy Oncology Group (RTOG) 9410 (abstract 1891). Proc Am Soc Clin Oncol 19:484a, 2000.
9. Curran WJ, Scott CB, Langer CJ, et al: Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III nsclc: RTOG 9410 (abstract 2499). Proc Am Soc Clin Oncol 22:621, 2003.
10. Byhardt RW, Scott CB, Ettinger DS, et al: Concurrent hyperfractionated irradiation and chemotherapy for unresectable nonsmall cell lung cancer. Results of Radiation Therapy Oncology Group 90-15. Cancer 75:2337-2344, 1995.
11. Lee JS, Scott C, Komaki R, et al: Concurrent chemoradiation therapy with oral etoposide and cisplatin for locally advanced inoperable non-small-cell lung cancer: Radiation therapy oncology group protocol 91-06. J Clin Oncol 14:1055-1064, 1996.
12. Movsas B, Scott C, Curran W, et al: A quality-adjusted time without symptoms or toxicity (QTWiST) analysis of Radiation Therapy Oncology Group (RTOG) 94-10 (abstract 1247). Proc Am Soc Clin Oncol 20:313a, 2001.
13. Furuse K, Fukuoka M, Kawahara M, et al: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17:2692-2699, 1999.
14. Fournel P, Maurice P, Gilles R, et al: A randomized phase III trial of sequential chemoradiotherapy versus concurrent chemo-radiotherapy in locally advanced non small cell lung cancer (NSCLC) (GLOT-GFPC NPC 95-01 study) (abstract 1246). Proc Am Soc Clin Oncol 20:312a, 2001.
15. Forastiere AA, Berkey B, Maor M, et al: Phase III trial to preserve the larynx: Induction chemotherapy and radiotherapy versus concomitant chemoradiotherapy versus radiotherapy alone, Intergroup Trial R91-11 (abstract 4). Proc Am Soc Clin Oncol 20:2a, 2001.
16. Fu KK, Pajak TF, Trotti A, et al: A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys 48:7-16, 2000.
17. Erdi YE, Rosenzweig K, Erdi AK, et al: Radiotherapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother Oncol 62:51-60, 2002.
18. Hoekstra CJ, Stroobants SG, Hoekstra OS, et al: The value of [18F]fluoro-2-deoxy-Dglucose positron emission tomography in the selection of patients with stage IIIA-N2 nonsmall cell lung cancer for combined modality treatment. Lung Cancer 39:151-157, 2003.
19. Pandit N, Gonen M, Krug L, et al: Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging 30:78-84, 2003
20. MacManus MP, Hicks RJ, Ball DL, et al: Imaging with F-18 FDG PET is superior to TI-201 SPECT in the staging of non-small cell lung cancer for radical radiation therapy. Australas Radiol 45:483-490, 2001.
21. Choi NC, Fischman AJ, Niemierko A, et al: Dose-response relationship between probability of pathologic tumor control and glucose metabolic rate measured with FDG PET after preoperative chemoradiotherapy in locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 54:1024-1035, 2002.
22. Willner J, Baier K, Caragiani E, et al: Dose, volume, and tumor control predictions in primary radiotherapy of non-small cell lung cancer. Int J Radiat Oncol Biol Phys 52:382- 389, 2002.
23. Curran WJ Jr, Scott C, Bonomi P, et al: Initial report of locally advanced multimodality protocol (LAMP): ACR 427: A randomized 3- arm phase II study of paclitaxel (T), carboplatin (C), and thoracic radiation (TRT) for patients with stage III non-small cell lung cancer (NSCLC) (abstract 1244). Proc Am Soc Clin Oncol 20:312a, 2001.
24. Choy H, Curran WJ Jr, Scott CB, et al: Preliminary report of locally advanced multimodality protocol (LAMP): ACR 427: A randomized phase II study of three chemo-radiation regimens with paclitaxel, carboplatin, and thoracic radiation (TRT) for patients with locally advanced non small cell lung cancer (LA-NSCLC) (abstract 1160). Proc Am Soc Clin Oncol 21:291a, 2002.
25. Saunders M, Dische S, Barrett A, et al: Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: A randomised multicentre trial. Lancet 350:161- 165, 1997.
26. Bailey AJ, Parmar MKB, Stephens RJ, et al: Patient-reported short-term and long-term physical and psychological symptoms: Results of the continuous hyperfractionated accelerated radiotherapy (CHART) randomized trial in non-small-cell lung cancer. J Clin Oncol 16:3082-3093, 1998.
27. Mehta MP, Tannehill SP, Adak S, et al: Phase II trial of hyperfractionated accelerated radiation therapy for nonresectable non-smallcell lung cancer: results of Eastern Cooperative Oncology Group 4593. J Clin Oncol 16:3518-3523, 1998.
28. Saunders MI, Rojas A, Lyn BE, et al: Dose-escalation with CHARTWEL (continuous hyperfractionated accelerated radiotherapy week-end less) combined with neo-adjuvant chemotherapy in the treatment of locally advanced non-small cell lung cancer. Clin Oncol 14:352-360, 2002.
29. Ginsberg RJ, Vokes EE, Rosenzweig K: Non-small cell lung cancer, in DeVita VT, Hellman S, Rosenberg SA (eds): Cancer: Principles & Practice of Oncology (6th ed), pp 925- 983. Philadelphia, Lippincott Williams & Wilkins, 2001.
30.Belderbos JSA, De Jaeger K, Heemsbergen WD, et al: First results of phase I/II escalation trial in non-small cell lung cancer using three-dimensional conformal radiotherapy. Radiother Oncol 66:119-126, 2003.
31. Xu SJ, Shi YS, Song HC, et al: Therapeutic effect of high-dose three-dimensional conformal radiotherapy and conventional radiotherapy for non-small-cell lung cancer. Di Yi Jun Yi Da Xue Xue Bao 22:937-938, 2002.
32. Singh AK, Lockett MA, Bradley JD, et al: Predictors of radiation-induced esophageal toxicity in patients with non-small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 55:337-341, 2003.
33. Sim S, Rosenzweig KE, Schindelheim R, et al: Induction chemotherapy plus threedimensional conformal radiation therapy in the definitive treatment of locally advanced nonsmall- cell lung cancer. Int J Radiat Oncol Biol Phys 51:660-665, 2001.
34. Socinski MA, Rosenman JG, Halle J, et al: Induction carboplatin (C), irinotecan (I), and paclitaxel (P) followed by concurrent CP and dose-escalated thoracic conformal radiation therapy (TCRT) in stage IIIA/B non-small cell lung cancer (NSCLC) (abstract 1266). Proc Am Soc Clin Oncol 21:317a, 2002.
35. Brodin O, Wagenius G, Helsing M: Daily paclitaxel (P) and radiotherapy (RT) in patients (Pts) with locally advanced non-small cell lung cancer (NSCLC). A phase I study (abstract 2022). Proc Am Soc Clin Oncol 19:516a, 2000.
36. Gasper L, Gandara D, Chansky K, et al: Consolidation docetaxel following concurrent chemoradiotherapy in pathologic stage IIIb non-small cell lung cancer (NSCLC) (SWOG 9504): Patterns of failure and updated survival (abstract 1255). Proc Am Soc Clin Oncol 20:315a, 2001.
37. Vokes EE, Herndon JE II, Crawford J, et al: Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB nonsmall- cell lung caner: Cancer and Leukemia Group B study 9431. J Clin Oncol 20:4191- 4198, 2002.
38. Fosella FV, Zinner RG, Komaki R, et al: Gemcitabine (G) with concurrent chest radiation (XRT) followed by consolidation chemotherapy with gemcitabine plus cisplatin (CDDP): A phase I trial for patients with stage III non-small cell lung cancer (NSCLC) (abstract 243). Proc Am Soc Clin Oncol 20:312a, 2001.