An Exploratory Study of the Combination of Monoclonal Antibodies Alemtuzumab and Rituximab in the Treatment of CD52- and CD20-Positive Chronic Lymphoid Disorders
Monoclonal antibodies demonstrate impressive response rates in lymphoid diseases, and treatment indications are expanding. Both alemtuzumab (Campath) (anti-CD52) and rituximab (Rituxan) (anti-CD20) are active in chronic lymphocytic leukemia (CLL) and low-grade lymphomas.
Monoclonal antibodies demonstrate impressive response rates in lymphoiddiseases, and treatment indications are expanding. Both alemtuzumab (Campath)(anti-CD52) and rituximab (Rituxan) (anti-CD20) are active in chroniclymphocytic leukemia (CLL) and low-grade lymphomas. However, activity in somesites (lymph nodes, liver, spleen) is limited. Here, we explore the safety andefficacy of a combination of alemtuzumab and rituximab in patients withrelapsed/refractory hematologic malignancies expressing CD52 and CD20.
Rituximab was given at a dose of 375 mg/m² IV every week × 4 doses.Alemtuzumab was given at a dose of 3, 10, and 30 mg IV on days 3, 4, and 5 ofweek 1, respectively. During weeks 2 through 4, alemtuzumab was given at a doseof 30 mg IV on days 3 and 5 of each week (total of seven 30-mg doses).Trimethoprim and sulfamethoxazole and valacyclovir (Valtrex) were given forprophylaxis.
A total of 31 patients have been treated (18 CLL, 8 CLL/prolymphocyticleukemia [PLL], 2 Richter’s syndrome, 3 mantle cell lymphoma). The median ageis 60 years (range: 42-79 years). The median number of previous treatments is4 (range: 1-8); 17/29 patients (59%) were refractory to fludarabine, 18/28(64%) to alkylators, and 14/27 (52%) to both; and 23/31 patients (74%) were Raistage ³ 3. The median beta-2-microglobulin level was 5.3 mg/dL (range: 2.4-15.8mg/dL). Five patients (2 CLL, 3 CLL/PLL) received two courses.
Toxicities were mainly grade 1/2 and infusion-related. Most frequentlyobserved were fevers and rigors (up to 97% of patients), dyspnea (52%),rash/hives (42%), fatigue (39%), myalgias (35%), and nausea/vomiting (23%).Responses by site (³ 50% improvement at each site) aregiven below:
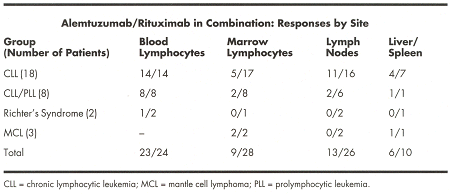
By National Cancer Institute response criteria,3 patients achieved a complete response (2 CLL,1 CLL/PLL), 10 patients a partial response (7 CLL,3 CLL/PLL), and 1 patient a nodal partial response (CLL), for an overallresponse rate of 45% (14/31 patients). Among responders, the median time toprogression was 7 months (range: 1-12 months) and the median survival was 9months (range: 3-13 months). Infections occurred in 15/31 patients, including5 cytomegalovirus (CMV) antigenemia, 5 pneumonia (1 viral, 2 fungal, 1 bacterial), 1 skin, and 7 fever of unknownorigin.
CONCLUSION: We conclude that the combination of alemtuzumab and rituximab inthis schedule is safe and shows encouraging activity in a poor-prognosis groupof patients.