FDA Approves Nexavar for Use in Advanced Kidney Cancer
The Food and Drug Administration (FDA) has approved Nexavar (sorafenib tosylate) tablets for the treatment of patients with advanced renal cell carcinoma. Nexavar, a multikinase inhibitor that has been shown to double progression-free survival in these patients, is the first FDA-approved treatment for this type of cancer in more than a decade, Bayer Pharmaceuticals Corporation and Onyx Pharmaceuticals, Inc.
WEST HAVEN, ConnecticutThe Food and Drug Administration (FDA) has approved Nexavar (sorafenib tosylate) tablets for the treatment of patients with advanced renal cell carcinoma. Nexavar, a multikinase inhibitor that has been shown to double progression-free survival in these patients, is the first FDA-approved treatment for this type of cancer in more than a decade, Bayer Pharmaceuticals Corporation and Onyx Pharmaceuticals, Inc. (Emeryville, California), said in a news release.
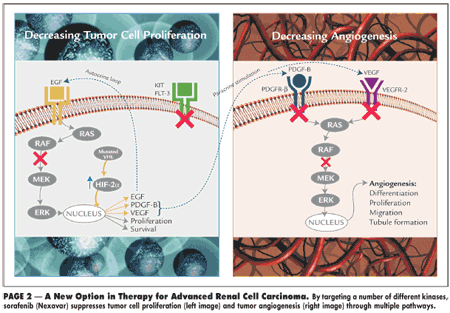
"Nexavar is an oral anticancer drug that blocks tumor growth in new ways," said Arthur Higgins, chairman of Bayer Healthcare's executive committee. In preclinical models, Nexavar was shown to target members of classes of kinases known to be involved in both tumor cell proliferation and tumor angiogenesis. These kinases include RAF kinase, VEGFR-2, VEGFR-3, PDGFR-?, KIT, and FLT-3.
12 Years of Work
Hollings C. Renton, chairman, president and CEO of Onyx, said that "the approval of Nexavar reflects the culmination of 12 years of hard work by countless dedicated scientists and clinicians, as well as the selfless participation of individuals suffering from advanced kidney cancer. We thank all of these groups for their important contributions to Nexavar's development."
The companies also announced that a new program, the Resources for Expert Assistance and Care Helpline (REACH), is available to answer questions about Nexavar treatment, reimbursement, and patient support (call 1-866- 639-2827).
"As a kidney cancer survivor, I'm pleased that there is a new treatment available for patients with this deadly diseasea disease for which additional treatment options are welcome," commented Bill Bro, president of the Kidney Cancer Association. "Breakthroughs in research over the last several years have given renewed hope to patients with advanced kidney canacer who previously had few treatment options."
Phase III Trial
Nexavar's FDA approval was based on phase III data from the largest randomized placebo-controlled trial ever conducted in patients with advanced renal cell cancer, the companies said. The international phase III study was reported at the 2005 American Society of Clinical Oncology (ASCO) meeting by Bernard Escudier, MD, of the Gustave-Roussy Institute, Paris (see ONI July 2005, page 1). The study involved 905 randomized patients with histologically or cytologically confirmed unresectable and/or metastatic renal cell cancer of clear cell histology.
Patients had to have measurable disease and failure to respond to one prior systemic therapy in the 8 months preceding enrollment. Nearly all the patients had had a nephrectomy, and the median time from their cancer diagnosis was about 2 years. The patients were randomized to receive either sorafenib 400 mg twice daily or placebo.
A total of 769 patients were included in Dr. Escudier's interim report. Because of the strong results in the interim analysis, the study was modified in April 2005 to allow patients to cross over from placebo to Nexavar.
Nexavar doubled progression-free survival to a median of 6 months, compared with 3 months for patients receiving placebo (hazard ratio 0.44, P < .000001). All subgroups examined, including patients who had not received conventional treatment with biologics, such as interleukin-2 or interferon-alfa, appeared to benefit as well.
At the time of a planned interim survival analysis, based on 220 deaths, overall survival was longer for Nexavar than placebo with a hazard ratio of 0.72, Dr. Escudier reported at the 13th European Cancer Conference (ECCO) in November 2005. This analysis did not meet the prespecified criteria for statistical significance, and additional analyses are planned at the time survival data mature when 540 deaths have occurred.
Well Tolerated
Nexavar was well tolerated. In the pivotal phase III trial, the most common reported treatment-emergent adverse events of any severity were diarrhea, rash/desquamation, fatigue, hand-foot skin reaction, alopecia, nausea, pruritus, hypertension, vomiting, and anorexia. Grade 3 treatment-emergent adverse events were reported in 31% of Nexavar patients vs 22% of placebo patients, and grade 4 events were reported in 7% vs 6%, respectively.
Nexavar has been studied in more than 20 tumor types and in more than 4,000 patients to date, the companies said. It is currently in phase III clinical trials for the treatment of advanced hepatocellular carcinoma and metastatic melanoma. In addition, a phase III clinical trial in non-small-cell lung cancer is planned for the first half of 2006.
As part of Bayer and Onyx's global registration strategy for Nexavar, a Marketing Authorization Application (MAA) was submitted to the European Agency for the Evaluation of Medicinal Products (EMEA) in London in September 2005, the companies said. In addition, they said, filings for Nexavar have been completed in Switzerland, Australia, Brazil, Canada, and Mexico.