Fertility-Preserving Options for Cervical Cancer
Childbearing is one of the most important life goals for many women, and fertility preservation is a very important factor in the overall quality of life of cancer survivors. Cervical cancer frequently affects young women; because some women tend to delay childbearing, fertility preservation must be considered when treatment options are discussed. Over the past decade, the radical trachelectomy procedure has become a well established fertility-preserving option for young women with early-stage cancer; this procedure is associated with low morbidity, good oncologic outcome, and a high proportion of pregnancies that reach the third trimester and babies that are delivered at term. This article will review available literature on the vaginal radical trachelectomy procedure and data from other surgical approaches, such as the abdominal radical trachelectomy. In addition, the potential future application of neoadjuvant chemotherapy followed by fertility-preserving surgery in patients with locally advanced cervical cancer will be examined. Finally, ultraconservative surgical approaches (eg, conization alone with or without laparoscopic lymphadenectomy) in very early-stage disease will be discussed.
Childbearing is one of the most important life goals for many women, and fertility preservation is a very important factor in the overall quality of life of cancer survivors. Cervical cancer frequently affects young women; because some women tend to delay childbearing, fertility preservation must be considered when treatment options are discussed. Over the past decade, the radical trachelectomy procedure has become a well established fertility-preserving option for young women with early-stage cancer; this procedure is associated with low morbidity, good oncologic outcome, and a high proportion of pregnancies that reach the third trimester and babies that are delivered at term. This article will review available literature on the vaginal radical trachelectomy procedure and data from other surgical approaches, such as the abdominal radical trachelectomy. In addition, the potential future application of neoadjuvant chemotherapy followed by fertility-preserving surgery in patients with locally advanced cervical cancer will be examined. Finally, ultraconservative surgical approaches (eg, conization alone with or without laparoscopic lymphadenectomy) in very early-stage disease will be discussed.
In 2002, cervical cancer was the fourth most frequent cancer diagnosed in women between 15 and 39 years of age, with only breast cancer, melanoma, and thyroid cancer diagnosed more frequently.[1] According to the Surveillance, Epidemiology, and End Result (SEER) data, cervical cancer affects 1 in 128 women in their lifetimes, representing approximately 10,520 new cases in the United States annually.[2] In the year 2000, nearly 28% of all patients diagnosed with cervical cancer and almost 39% of patients with stage I disease were less than 40 years old.[3] Thus, a significant proportion of women with early-stage disease are diagnosed during their childbearing years.
A definite trend toward deferring childbearing into the late 30s and early 40s has been noted, particularly in Western countries. Indeed, between 1990 and 2002, the incidence of first births among women 35 to 39 years of age has increased by 31% and, for women 40 to 45 years old, by 51%.[4] Luckily, the cure rate for early-stage cervical cancer is excellent; the 5-year survival rate is 92%, although standard oncologic treatment leads to permanent infertility in almost all cases. Thus, a significant proportion of women will be diagnosed with the disease before they have had the chance to have children, and, since the overall prognosis is so good, the issue of fertility preservation becomes of paramount importance when discussing treatment options with these young patients.
Over the last 15 years, radical trachelectomy has been recognized as a valuable fertility-preserving option for young women with early-stage disease. For a long time, clinicians believed that very few women would be candidates for this procedure. Sonoda and others recently published an interesting 16-year study examining all women undergoing a radical hysterectomy to treat early-stage cervical cancer at Memorial Sloan-Kettering Cancer Center.[5] In all, 43% (186/435) were under age 40 and potentially were interested in fertility preservation; of these, 48% would have met the selection criteria to undergo a radical trachelectomy. These results indicated that a substantial proportion of patients with early-stage disease may qualify for this fertility-preserving procedure and, therefore, should be counseled about this option preoperatively.
Thus, although the subject has been neglected for a long time, fertility preservation is now considered to be an important quality-of-life issue for young patients with early-stage cervical cancer, and it is being studied in a more systematic and comprehensive way. After initiating cancer treatment, women often regret not having received all the appropriate information needed for decisions on fertility preservation options. In examining the psychosocial impact of cancer-related infertility in women treated for gynecologic malignancies, Carter et al discovered that a high proportion of these women experience depression, grief, stress, and sexual dysfunction.[6] Further, women faced with loss of fertility from gynecologic cancer treatments believed that they were deprived of a choice and, sadly, that medical professionals tended to minimize their sense of loss.[7]
In this article, we will review surgical options to preserve fertility and emphasize the vaginal radical trachelectomy procedure and its oncologic, obstetric, and fertility outcomes. In addition, other radical trachelectomy techniques will be discussed, with the advantages and disadvantages of each highlighted. Even more ultraconservative approaches, such as conization with or without lymphadenectomy for very early-stage disease, also will be reviewed. Finally, new options to preserve fertility in locally advanced cervical cancer that integrate neoadjuvant chemotherapy and fertility-preserving surgery will be examined.
Vaginal Radical Trachelectomy
At the end of the 1980s, Dargent developed the vaginal radical trachelectomy procedure, which involves a laparoscopic pelvic lymph node dissection followed by the removal of the cervix and proximal parametrial tissue.[8] The body of the uterus is retained to preserve fertility. The technical aspects of the procedure have been detailed elsewhere.[9]
The popularity of the radical trachelectomy procedure came somewhat slowly. However, it is gaining wider acceptance among gynecologic oncologists as the oncologic, obstetric, and fertility data available in the literature become more substantial and promising.
Oncologic Results
Selection Criteria-A set of criteria to select candidates for this procedure was proposed in 1998.[10] Most of these criteria have remained unchanged, except, perhaps, for tumor size. The procedure is generally limited to women with a lesion smaller than 2.0 to 2.5 cm in size, but it may be possible in patients with larger lesions, particularly if they are very exophytic.
Magnetic resonance imaging (MRI) is useful in evaluating patients for this procedure preoperatively. In particular, detection of an endocervical extension of the tumor in relation to the internal os may help in the preoperative exclusion of patients who have more extensive disease and are unlikely to have free margins at the time of the trachelectomy.[11] Peppercorn et al found that the sensitivity of the MRI in assessing the relation of the tumor to the internal os was 100%, and the specificity was 92%.[11] Further, MRI provides precise measurements of tumor diameter and volume and may detect early parametrial invasion.[12,13]
Occasionally, myometrial extension-which is likely to be missed on clinical evaluation-also may be detected using MRI. Although this test may assess lymph node status, it may miss metastasis. Bellomi et al recently reported that the sensitivity and specificity of MRI with respect to accurately diagnosing lymph node metastasis were 73% and 93%, respectively, and the positive predictive value was only 53%.[14]
In our series and in most others, the rate of abandoning planned radical trachelectomies because of detection of more advanced disease (eg, positive lymph nodes, inadvertent discovery of more extensive endocervical extension) during the procedure was about 10%.[15] In the latter situation, a completion radical vaginal hysterectomy may be performed immediately after trachelectomy. Thus, it is highly important to obtain preoperative permission from patients for radical hysterectomy, should it become necessary.
Recurrences-Clearly, a careful preoperative investigation is critical to rule out patients who are not candidates for this procedure, reduce the chances of abandoning the procedure intraoperatively, and lower the risk of recurrence. As more data on recurrences are reported, risk factors for recurrences may become better defined.
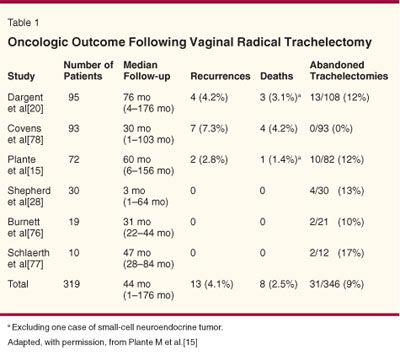
Table 1 presents a summary of recurrences following vaginal radical trachelectomy from six published series.[15] The data indicate that the overall recurrence rate is less than 5% and the death rate is 2.5%, which are similar to the recurrence and death rates following radical hysterectomy for early-stage disease. In our series, the actuarial 5-year disease survival was 95%.[15] The sites of recurrences were variable, with half being in the pelvis (parametrium and pelvic sidewall) and the others being at distant sites (intra-abdominal and in the para-aortic/supraclavicular nodes). In comparing the survival of patients following a radical trachelectomy to patients with similar-sized lesions treated with standard radical hysterectomy, Covens et al found no significant difference in survival.[16]
Recently, three additional local recurrences following vaginal radical trachelectomy have been reported. One occurred in the bladder and iliac nodes of a patient 26 months after she underwent trachelectomy. The lesion was a stage IB1 adenocarcinoma measuring 2.1 cm × 2.0 cm. All 30 lymph nodes were negative, but the surgical margins were only free by 5 mm.[17] The second occurred in the rectovaginal and vesicovaginal space 4 years after the trachelectomy. This stage IB1 squamous lesion measured 1.5 cm; the 14 lymph nodes were negative. The surgical margins were clear by > 10 mm, but isolated vascular space invasion were present.[18] In the third case, an adenocarcinoma was diagnosed at the level of the cervix 7 years following trachelectomy despite regular 6-month follow-ups.[19] However, whether this last lesion represented a true recurrence or a secondary primary tumor is questionable.
Prognostic Factors-The available data suggest that larger lesions may be associated with a higher risk of recurrence. Indeed, our data and those from Dargent indicated that lesions larger than 2 cm may pose a statistically higher risk of recurrence.[20] Vascular space invasion also may be a risk factor for recurrence. Currently, however, it is not considered to be a contraindication for the procedure. Histology is not associated with recurrences. Further, even though adenocarcinomas involve the endocervix more frequently, they currently do not appear to be clearly associated with a higher risk of recurrence.
Another important prognostic factor is lymph node status-intraoperative detection of lymph node involvement greatly jeopardizes the option for a conservative treatment approach. Thus, we favor abandoning this procedure in favor of instituting combined chemoradiation. Para-aortic nodes may be sampled laparoscopically to rule out metastasis at that level, and ovaries may be transposed to preserve some ovarian function and avoid/delay menopause despite radiation therapy. Intraoperatively, the sentinel node mapping technique may help to detect lymph node metastasis by directing the frozen section analysis to the lymph nodes most likely to be involved.[21,22] Discovering positive nodes postoperatively is always devastating; therefore, the clinician should make the greatest effort to detect lymph node metastasis preoperatively or intraoperatively.
Follow-up-The follow-up of women after trachelectomy is similar to that of patients who have undergone hysterectomy-they must have visits every 3 months for the first 2 years, followed by visits every 4 to 6 months for the next 3 years and then annual examinations. Ideally, a colposcopic evaluation should be performed along with a cytology. Use of the cytobrush may help to better sample the endocervical canal in patients having small cervical openings. Long-term follow-up is very important, since late recurrences or new primaries may develop several years after the trachelectomy.[19] However, no available evidence suggests that a complete hysterectomy needs to be performed after a patient's family is complete; this decision remains at the discretion of patients and their physicians.
Following the trachelectomy, the relative cervical stenosis may make the colposcopic evaluation unsatisfactory, and the cytology may become difficult to obtain and to interpret. Singh et al reviewed 197 smears from 32 women who underwent a radical trachelectomy.[23] They noted that endometrial cells were present in up to 58% of the smears and led to false-positive malignant reports in 2%. A high proportion of smears contained only squamous cells without glandular cells and were considered unsatisfactory. Investigation of the patients with unsatisfactory smears or those with "atypical" changes often does not reveal anything, but two patients developed pelvic recurrences, and, in both cases, cytologic anomalies were present long before the recurrence was clinically or radiologically confirmed.[23]
Thus, cytologic abnormalities found post-trachelectomy must be taken seriously and worked up accordingly, particularly when they are persistent. In our series, we found several patients who had various degrees of atypical cells on smears that usually were of glandular origin. Presumably, these represented inflamed endometrial cells from the lower uterine segment. After thorough evaluation, however, none have turned out to be malignant. A human papillomavirus DNA test may help in determining the significance of cytologic anomalies.
An MRI can also be very useful following patients post-trachelectomy to detect pelvic tumor recurrences, although no guidelines concerning the frequency of such testing in post-trachelectomy patients are available.[24] Sahdev et al recently reviewed anatomic changes associated with the procedure, such as the appearance of the end-to-end anastomosis between the uterus and the vaginal vault, neofornix formation of the posterior vaginal wall, hematomas, suture/cerclage artifacts, isthmic stenosis, exaggeration of the pelvic plexuses, and thickening of the vaginal walls.[24] Clinicians and radiologists must recognize such anatomic changes, as they may easily be misinterpreted as recurrences.
Obstetric Results
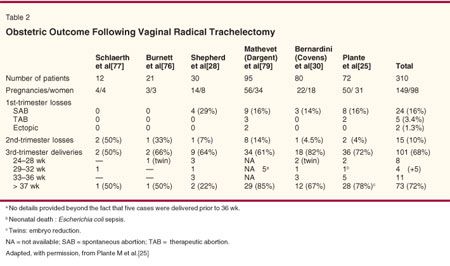
Published obstetric data indicate that pregnancy outcomes following a vaginal trachelectomy are quite acceptable. A summary of the published outcomes from the same six series previously discussed, which involved almost 100 pregnancies, recently was updated (Table 2).[25]. One-third of the pregnancies ended in the first or second trimester, yet two-thirds reached the third trimester. Of those, one-quarter of the patients delivered prematurely (< 37 weeks), and three-quarters delivered at term. Another recent review of 16 series involving 161 pregnancies post-trachelectomy yielded similar results.[26]
First-Trimester Losses-Overall, the rate of first-trimester miscarriage is approximately 16%, which is comparable to that found in the general population. Luckily, most miscarriages can be managed expectantly. If a curettage is needed, minimal cervical dilatation should be achieved to avoid breaking the cerclage; if necessary, another cerclage can be placed at the time of the next pregnancy at 14 to 16 weeks of gestation.
In our series, six of the eight miscarriages were managed conservatively, and five spontaneous abortions occurred; one patient required misoprostol, but a curettage was needed because of an incomplete evacuation and subsequent endometritis. Conversely, the other two patients were induced readily with misoprostol; one miscarried spontaneously, and the other required a curettage.
Second-Trimester Losses-The rate of second trimester loss is actually higher than that of the general population (10% vs 4%, respectively), and its management is often challenging. Conservative management should be attempted first, if possible. In our series, the two cases aborted spontaneously; one patient delivered spontaneously at 17 weeks of gestation from chorioamnionitis following a genetic amniocentesis, and the cerclage did not appear to cause a problem.
If the membranes rupture or the cervix opens, a trial of labor induction with misoprostol may be attempted with or without removal of the cerclage to allow vaginal delivery.[16] If this is unsuccessful, a dilatation and evacuation may be performed with or without removal of the cerclage. Ultimately, if the mother's condition deteriorates or if other conservative measures fail, a hysterotomy may be needed.
Third-Trimester Deliveries-Our review of obstetric outcomes from 100 deliveries indicates that nearly two-thirds of the pregnant women in this series have reached the third trimester.[25] Roughly 25% of these have delivered prematurely (ie, < 37 weeks of gestation). Only 10% have delivered with significant prematurity (< 28 weeks of gestation) usually related to perinatal mortality and morbidity. This group contained three sets of twins-a matter of concern, as these pregnancies already are at much higher risk for premature delivery.
Conversely, 75% of the pregnant patients who reached the third trimester delivered at term by elective cesarean section. There is no evidence that the newborns are small for gestational age, as supported by the recent study by Klemm et al, who showed by transvaginal Doppler sonography that the uterine perfusion after radical vaginal trachelectomy remains unchanged.[27]
Technically, the cerclage could be removed, and vaginal delivery could be allowed, but the short and scarred cervix may not dilate well. Further, a tear that occurs at the time of delivery may be difficult to repair or may lead to significant bleeding if it extends up to the uterine vessels. Also, if a patient wishes to have more than one pregnancy, it may be preferable not to remove the cerclage.
It is reassuring to know that it is possible to carry several pregnancies to term following a trachelectomy. Indeed, in our series, 16% of the patients had up to four pregnancies following the trachelectomy.[25]
Preterm Births and Second Trimester Losses-Preterm births and second trimester losses are serious concerns in pregnant women who have undergone radical trachelectomies. Two etiologies have been proposed to explain this phenomenon.[28] The first is mechanical in nature and focuses on the lack of lower uterine segment support caused by a very short cervix and resulting cervical incompetence. The second etiology is infectious in nature and probably more important-a shortened cervix may lead to an inadequate or insufficient mucus plug, which is an important natural barrier against ascending infections. This inadequacy may lead to chronic subacute chorioamnionitis, which eventually activates the cytokine cascade and leads to premature rupture of the membranes; premature labor usually occurs secondarily. It is unclear whether the cerclage itself may be a source of bacterial contamination, although a case of Actinomyces infection associated with the cerclage has been reported.[29]
If the membranes rupture prematurely, usually as a result of an underlying chorioamnionitis, there is no benefit in postponing the delivery after administration of a steroid injection and antibiotics.[25,30,31] Most patients usually begin labor within 48 hours, and delays may increase the risk of neonatal and maternal sepsis. In our series, one newborn died of fulminant Escherichia coli sepsis that might have been prevented if the child had been delivered more rapidly.[25] In cases of premature rupture of membranes in the general obstetric population, expectant management beyond 34 weeks of gestation is of limited benefit, with antibiotic administration beneficial to both the mother and the neonate.[32,33]
Management of Pregnancies Post-trachelectomy-General obstetric literature defines a short cervix as measuring less than 2.5 cm; women with a short cervix have a ninefold increase in the risk of delivering before 35 weeks of gestation.[34] As mentioned previously, women who have undergone trachelectomy have a higher risk of premature delivery, considering that they have very short cervices. There are no published guidelines for managing pregnant women following a radical trachelectomy, and all of the following subjects should be discussed with a perinatal specialist. That said, we recently proposed some basic recommendations based on our experience and largely extrapolated from the literature on premature rupture of the membranes and incompetent cervix in the general obstetric population.[25,35,36]
To reduce the risk of introducing infections, routine cytologies probably should be avoided throughout the first trimester and even throughout the pregnancy, unless there is a particular concern. Sexual intercourse may be a source of infection; patients are advised to avoid coitus from the 20th to the 36th week of pregnancy. The value of routine prophylactic antibiotic use between the 14th and 16th weeks of gestation is unclear, as is Shepherd's recommendation that routine vaginal cultures be performed every 2 weeks throughout pregnancy.[28] In addition, it is not clear whether routine prophylactic steroid coverage (to accelerate fetal lung maturity in view of the higher risk of premature delivery) is warranted.[28]
The issue of the Saling procedure has been debated.[37] According to Mathevet and others, this procedure, which involves complete coverage of the cervix os by advancing a strip of vaginal mucosa over the cervical opening to prevent ascending infection, decreases the rate of preterm delivery.[38] However, the procedure itself, which is usually carried out at 14 weeks of pregnancy, poses some possible complications. We do not perform the Saling procedure on a routine basis; however, we reserve it as an option for women who have had a premature delivery at their first pregnancy.
Digital cervical examinations should be kept at a minimum to reduce the risk of introducing infection; these examinations may be replaced by transvaginal ultrasound (TVU), a technology that has been superior in assessing cervical length.[39] Particularly between weeks 14 and 24 of gestation, serial TVU appears to be a good predictor of preterm delivery in high-risk pregnancies.[40]
Several recent studies have demonstrated that progesterone suppositories are useful in reducing the risk of preterm birth in high-risk women, such as those with a previous preterm birth or those with a cerclage.[41-43] No data on their role and efficacy in pregnant women post-trachelectomy are available, but patients may be offered this option based on the fairly strong data in nontrachelectomy patients. Another potentially valuable option is the intake of fish oil (eg, omega-3) as a source of fatty acid. According to Olsen et al, the tocolytic properties of this supplement are probably mediated by the reduction of prostaglandin activity and can reduce recurrence of preterm delivery.[44]
Clearly, pregnant women who undergo radical trachelectomy are at higher risk of preterm delivery either from a mechanical or infectious etiology. Such patients may be followed by experts in cases of high-risk premature rupture of the membranes or an incompetent cervix.
Fertility Outcome
When radical trachelectomy was initially employed, clinicians and patients had concerns regarding the ability of women to conceive following the procedure. Experience shows that most women can subsequently become pregnant naturally without assisted reproductive technologies.[25,26] Women who do not become pregnant naturally need a thorough infertility evaluation to identify the cause of infertility (eg, an ovulatory problem, tubal factor, endometriosis, male infertility).
If no cause is identified, then the infertility may be related to the cervix secondary to the trachelectomy procedure (eg, cervical stenosis, fibrosis, inadequate mucus production). Many patients with infertility secondary to a cervical cause have conceived successfully following IUI or IVF.[25,30] With IVF, the risk of multiple gestations is particularly hazardous, especially considering an increased risk of preterm birth with twins and the reduced lower uterine support post-trachelectomy. One patient who conceived twins following IVF chose to undergo embryo reduction, and she delivered one healthy baby at term. She subsequently became pregnant spontaneously. In our series, five of the seven patients who could not conceive following the trachelectomy procedure managed to get pregnant either spontaneously or with IUI or IVF.[25]
A history of infertility should not be a contraindication to radical trachelectomy. Some patients with a history of infertility have conceived spontaneously or with the help of assisted reproductive technology after the trachelectomy.[16] Thus, fertility-preserving surgery should not be denied to these patients. Finally, women may not need a complete infertility work-up before undergoing radical trachelectomy, although some patients may wish to do so.
Cervical Stenosis-Cervical stenosis post-trachelectomy is an important issue. Some women may become symptomatic, usually presenting with cyclical abdominal pain and cramps. Cannulation of the cervical canal may be attempted under anesthesia, ideally when the patient is menstruating, to help identify the cervical opening. The cannulation may require the assistance of ultrasonic and laparoscopic assistance to avoid false passage and perforation.[45] The authors were recently successful in cannulating the cervix using small lacrymal probes.
Asymptomatic women may need no additional treatment. If the patient develops infertility problems that are thought to be secondary to the cervical stenosis and cannulation of the cervix, and intrauterine insemination (IUI) cannot be accomplished, in vitro fertilization (IVF) is probably the best option. However, embryo transfer may be difficult. In a recent report, Aust and others recounted their experience with a particularly difficult case. After identifying the cervical opening and dilating the cervical canal, they placed a Malecot catheter in the cervix to keep it open while the patient underwent ovarian induction.[46]
Alternative Surgical Approaches
Because of the natural anatomic accessibility of the cervix through the vagina, the initial surgical technique for the radical trachelectomy, as pioneered by Dargent, favored the vaginal approach. However, the education of surgeons and long learning curve needed to master the technique remains a significant obstacle in the widespread dissemination of the procedure, particularly in North America, where gynecologic oncologists do not frequently perform vaginal surgery. The procedure's popularity and accessibility has suffered from the limited acquisition of skills in laparoscopic and vaginal surgery. To circumvent some of those technical difficulties, alternative surgical approaches have been developed over the past 10 years.
Abdominal Radical Trachelectomy
In 1997, Smith et al first described two cases in which the abdominal radical trachelectomy was used.[47] The investigators described division of the uterine arteries and unsuccessful attempts made to reanastomose them. Later, the uterine arteries were transected, and the uterus apparently remained viable through the ovarian pedicles blood supply only.
The same group recently published their experience with 33 patients, 3 (9%) of whom were excluded because of positive nodes (2 patients) and a positive cervical margin (1 patient).[48] Interestingly, they included five patients with stage IB2 cervical cancer, with lesions reaching up to 6 cm in size. Nearly 66% of patients received a median of 2 units of blood, which is seldom required after a vaginal trachelectomy. Hospital stays ranged from 12 to 22 days, compared to 2 to 3 days needed following the vaginal approach. Just one ureteral injury was noted. Normal menstrual patterns resumed in all but two patients; ultrasound in one patient demonstrated an endometrial cavity consistent with Asherman's syndrome, and cervical stenosis requiring dilatation was noted in the other.[48] Only five patients have attempted to conceive thus far; three have succeeded, including one who required IVF. One patient miscarried in the first trimester, and two delivered neonates of good weight at term via cesarean section. Overall, the obstetric results, although limited, appear promising.[48]
In terms of oncologic outcome, the same group recently reported the first recurrence in a patient with a 3.8-cm, exophytic lesion.[49]. The nodes and margins were negative, there was less than one-third stromal invasion, and no vascular space invasion was noted. When seen 4 months after surgery, the patient had a normal exam, but she presented 2 months later with a 6-cm, right-sided, central vaginal recurrence. After treatment with chemoradiation, she now appears to be disease-free. The authors questioned whether this unusually rapid recurrence might have been secondary to "seeding" of cancer cells at the time of surgery.[49]
Another group recently reported a second, very early local recurrence on the cervix itself following an abdominal trachelectomy. This squamous cell carcinoma measured 4 × 10 mm with vascular space invasion but featured negative margins by 15 mm on the trachelectomy specimen. At 6-month follow-up, a cervical cytology showed suspicious cells, and the hysterectomy specimen showed an isolated 3-mm recurrence in the residual cervix.[50]
Another group reported their experience with abdominal radical trachelectomy in three women with cervical cancer.[51] One patient had a successful pregnancy, delivered at term, and was pregnant for a second time.
Another potential application of the abdominal trachelectomy is in the pediatric population, in whom the vaginal approach is impossible. Abu-Rustum et al reported on the successful management of two girls diagnosed with clear cell carcinoma of the cervix at ages 6 and 8 years.[52] It remains to be seen whether these young patients will have normal menses in the future and preservation of their fertility potential.
Advantages and Disadvantages-The main advantage of the abdominal radical trachelectomy is that the technique is almost identical to that of standard abdominal radical hysterectomy, except that the lower uterine segment is excised and reanastomosed to the vagina. Since it requires no skills in laparoscopic or vaginal surgery, the procedure is much easier to learn for gynecologic oncologists trained primarily in radical abdominal surgeries, with the learning curve much shorter and the strategy more familiar to gynecologic oncologists trained in the United States.
Conversely, Dargent would argue that surgical training-not the technique-must be modified.[53] Ungar et al indicated that an advantage of the abdominal approach is that more radical excision of the parametrium can be accomplished, and thus, the procedure can be performed on larger lesions.[48] In view of the recurrences recently reported, longer follow-up will be necessary to determine if radical trachelectomy is a safe option in patients with bulky cervical lesions. Whether wide parametrial excision is required for very early-stage disease is questionable, as the risk of parametrial extension is extremely low.[54]
Disadvantages of the abdominal approach include significant blood loss, high rate of blood transfusions, and the need for an abdominal incision related to the procedure. Ligation of the uterine arteries may lead to cervical/isthmic stenosis and amenorrhea. Longer follow-up will indicate whether the procedure has any impact on future pregnancies and birth weight.
Laparoscopic Radical Trachelectomy
Reflecting the evolution and skills gained in laparoscopic surgery, Lee and others reported on a modification of the laparoscopically assisted radical vaginal hysterectomy (LAVRH) that was used in two patients.[55] This approach requires extensive skills in laparoscopic surgery but limited skills in vaginal surgery.
Using this technique, 80% of the procedure is accomplished laparoscopically, and the rest is performed vaginally. The opening of the spaces and the division of the upper cardinal, uterosacral, and vesicouterine ligaments are all performed laparoscopically, whereas amputation of the cervix, division of the paracolpos, and reanastomosis to the lower uterine segment are accomplished vaginally. The two patients lost 900 and 400 mL of blood, respectively, and no intraoperative complications were noted.[55] Thus far, no pregnancies or recurrences have been reported.
Laparoscopic Abdominal Radical Trachelectomy
Given the trend toward more extensive laparoscopic surgery, Cibula et al recently reported on use of a complete laparoscopic trachelectomy, in which dissection is entirely laparoscopic, and the vagina is used only to remove the specimen.[56] This case report showed no surgical complications, and the blood loss was 250 mL. This approach requires advanced skills in laparoscopic surgery but no skills in vaginal surgery.
Evolution of the Technique
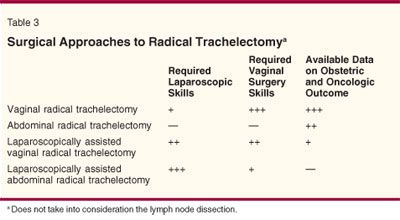
The evolution in the radical trachelectomy surgical techniques from a purely vaginal to a purely abdominal approach, with all shades and degrees of laparoscopic dissection in between, has been significant (Table 3). All of these techniques have advantages and disadvantages. More data are available on the vaginal approach, and more information about the safety profile and long-term results of the other approaches are needed. Until more information is available, surgeons must use the technique with which they are most comfortable.
Each of these procedures has merit. What matters most is that the procedure is well done, that the complication rate and the recurrence rate are low, that women retain their fertility potential, and, importantly, that women have access to the procedure-its availability should not be restricted to a few centers with skilled staff in extensive laparoscopic procedures and radical vaginal surgeries.
Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer
The radical trachelectomy is usually reserved for patients with early lesions measuring less than 2 cm, because the oncologic results are generally excellent. However, some patients are considered ineligible for this fertility-sparing procedure, either because their lesions exceed 2 cm in size or because they have more advanced local disease.
In the past decade, several authors have published their experience with the use of neoadjuvant chemotherapy followed by radical surgery in locally advanced cervical cancer. In general, neoadjuvant chemotherapy protocols include a multiagent, platinum-based chemotherapy regimen. A recent, randomized, Italian multicenter study in patients with stage IB2-IIB lesions showed a survival benefit for those who received neoadjuvant chemotherapy followed by radical surgery, compared with patients who received conventional radiotherapy.[57]A recent literature review supports this concept of chemotherapy after three cycles.[58]
Cervical cancer is clearly chemo-sensitive, and complete responses have allowed a few postchemotherapeutic pregnancies to occur. For instance, researchers reported an anecdotal case of a successful pregnancy following two cycles of primary chemotherapy (cisplatin and bleomycin) for a bulky stage IIA lesion. The response was complete and long-lasting.[59] Another group reported a partial response after two cycles of chemotherapy in a woman diagnosed with a 5-cm, stage IIB lesion at 14 weeks of pregnancy. Unfortunately, the patient declined further treatment and died of the disease. Nevertheless, at age 3, her child is developing normally.[60]
Still another group reported on two women diagnosed with locally advanced cervical cancer when they were in the second trimester; the women elected to receive chemotherapy and continue with the pregnancy. Both had a significant tumor response and underwent radical surgery at the time of cesarean section. No evidence of adverse fetal effects from the chemotherapy was found.[61]
Recently, an Italian group led by Maneo presented provocative preliminary data on the use of neoadjuvant chemotherapy followed by a conization and pelvic lymphadenectomy in young women with cervical cancers (lesions < 3 cm).[62] The chemotherapy regimen included a combination of paclitaxel, ifosfamide, and cisplatin, based on the protocol developed by the Monza group.[63] Of the 18 treated patients, 7 (39%) had either no residual disease or only in situ disease, 7 (39%) had minimal residual disease measuring < 3 mm in depth, and 4 (22%) had residual lesions between 3 and 10 mm deep. No patients showed disease progression under chemotherapy. Following treatment, eight women attempted to conceive; five succeeded, and six babies were born.[62] Following the same protocol, we recently treated three patients with lesions measuring 3 to 4 cm, and all had a complete pathologic response (ie, no residual disease on the trachelectomy specimen) after three cycles.[64]
Thus, fertility preservation is definitely possible after neoadjuvant chemotherapy, but long-term results are needed before conclusions can be drawn as to the safety and efficacy of this approach. These preliminary experimental data may lead to new approaches other than standard radiotherapy in managing locally advanced cervical cancer. Ovarian function may be preserved (particularly in young women), and premature menopause (as well as the adverse effects of radiation therapy on sexual function) may be avoided.
Ultraconservative Surgery for Very Early Lesions
At the other end of the spectrum, more conservative surgical treatments for very early lesions have been proposed because of their excellent prognosis. In early-stage disease, the high likelihood of finding no residual disease in a trachelectomy or hysterectomy specimen following analysis of a large diagnostic cone and the fact that parametrial tissues rarely are involved in very early-stage disease favor performance of less-extensive surgery.[15,54] In our series, up to 60% of patients had no evidence of residual cancer in their trachelectomy specimen.[15]
A few recent reports of conservative surgical procedures for very early-stage cervical cancer, such as excisional laser conizations and loop electrosurgical excision procedures (LEEPs), have been published.[65-68] More data are necessary to evaluate the oncologic safety of these ultraconservative approaches. The obstetric implications of large-cone biopsy or LEEP must be evaluated as well. One series concluded that LEEPs do not increase the rate of premature deliveries,[69] but other studies, including a recent large meta-analysis, found that the risk of preterm birth increases after LEEP procedures and conizations and that the depth of the cone may be related to adverse outcome in pregnancy.[70-72] Berghella et al followed women who had a prior cone biopsy or a LEEP with serial transvaginal ultrasound between 16 and 24 weeks of gestation.[73] They found that 30% of the women with a short cervix (< 2.5 cm) delivered prematurely (before 35 weeks of gestation), which is actually similar to the rate of preterm birth after a trachelectomy.[25,73]
The risk of microscopic spread to the parametrial tissue occurs rarely in patients with very early lesions.[54] However, the risk of lymph node metastasis remains a potential threat, even in stage IA1 tumors [74,75]. Particularly for stage IA2 lesions, the combination of cone biopsy with laparoscopic lymph node dissection, or at least with sentinel node mapping, may be safer.
Clearly, the key issues for these ultraconservative approaches will be careful patient selection and expert pathologic evaluation for accurate diagnosis of microinvasion. Adhering to strict selection criteria will be of paramount importance to avoid recurrence and death. Although ultraconservative treatment to preserve fertility is a provocative option, it should not jeopardize outcome.
Conclusions
The past decade has been an exciting time in the management of cervical cancer. Indeed, the development of advanced laparoscopic and vaginal surgery has revolutionized the surgical management of cervical cancer since the Wertheim radical hysterectomy era and has allowed the development of several fertility-preserving options for young women with early-stage disease.
The pioneering work of Dargent has forced the gynecologic oncology community into rethinking long-established concepts in managing this disease. Until now, fertility-preserving options have been reserved for women with very early-stage disease. In the future, multimodality treatment options that include neoadjuvant chemotherapy may offer new fertility-preserving alternatives to women with more advanced disease. Conversely, radical surgery with parametrial resection and node dissection may be too extensive in very early-stage disease, and conization with or without sentinel node mapping may be adequate. To collect data on those important and controversial issues, international collaborative efforts should be encouraged.
Now that new and more conservative surgical options are available to manage early-stage cervical cancer, gynecologic oncologists involved in caring for young cervical cancer patients must be aware of these new developments to offer full and comprehensive counseling. Careful patient selection and adequate surgical skills will be critical to obtaining a good outcome. As more authors report their experience with the various radical trachelectomy surgical approaches in the future, the advantages and disadvantages of each should become more clear.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. National Cancer Institute: Surveillance, Epidemiology and End Results. Available at http://seer.cancer.gov/. Accessed March 2, 2006.
2. Jemal A, Tiwari RC, Murray T, et al: American Cancer Society. Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004.
3. National Cancer Institute: Surveillance, Epidemiology and End Results. Cancer statistics review, 1975-2001. Available at http://seer.cancer.gov/csr/1975_2001/. Accessed March 2, 2006.
4. Martin JA, Hamilton BE, Sutton PD, et al: Births: Final data for 2002. Natl Vital Stat Rep 52:1-113, 2003.
5. Sonoda Y, Abu-Rustum NR, Gemignani ML, et al: A fertility-sparing alternative to radical hysterectomy: How many patients may be eligible? Gynecol Oncol 95:534-538, 2004.
6. Carter J, Rowland K, Chi D, et al: Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol 97:90-95, 2005.
7. Corney RH, Crowther ME, Everett H, et al: Psychosexual dysfunction in women with gynaecological cancer following radical pelvic surgery. Br J Obstet Gynaecol 100:73-78, 1993.
8. Dargent D, Brun JL, Roy M: La trachélectomie élargie (T.E.). Une alternative à l’hystérectomie radicale dans le traitement des cancers infiltrants développés sur la face externe du col utérin. J Obstet Gynecol 2:292-295, 1994.
9. Plante M, Renaud M-C, Roy M: Vaginal radical trachelectomy, in Levine DA, Barakat RR, Hoskins WJ (eds): Atlas of Procedures in Gynecologic Oncology, pp 207-221. London, Martin Dunitz, 2003.
10. Roy M, Plante M: Pregnancies after radical vaginal trachelectomy for early-stage cervical cancer. Am J Obstet Gynecol 179:1491-1496, 1998.
11. Peppercorn PD, Jeyarajah AR, Woolas R, et al: Role of MR imaging in the selection of patients with early cervical carcinoma for fertility-preserving surgery: Initial experience. Radiology 212:395-399, 1999.
12. Wagenaar HC, Trimbos JB, Postema S, et al: Tumor diameter and volume assessed by magnetic resonance imaging in the prediction of outcome for invasive cervical cancer. Gynecol Oncol 82:474-482, 2001.
13. deSouza NM, McIndoe GA, Soutter WP, et al: Value of magnetic resonance imaging with an endovaginal receiver coil in the pre-operative assessment of stage I and IIa cervical neoplasia. Br J Obstet Gynaecol 105:500-507, 1998.
14. Bellomi M, Bonomo G, Landoni F, et al: Accuracy of computed tomography and magnetic resonance imaging in the detection of lymph node involvement in cervix carcinoma. Eur Radiol 15:2469-2474, 2005.
15. Plante M, Renaud MC, Harel F, et al: Vaginal radical trachelectomy: an oncologically safe fertility-preserving surgery. An updated series of 72 cases and review of the literature. Gynecol Oncol 94:614-623, 2004.
16. Covens A, Shaw P, Murphy J, et al: Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer 86:2273-2279, 1999.
17. Morice P, Dargent D, Haie-Meder C, et al: First case of a centropelvic recurrence after radical trachelectomy: Literature review and implications for the preoperative selection of patients. Gynecol Oncol 92:1002-1005, 2004.
18. Morice P, Haie-Meder C, Pomel C, et al: Regarding "First case of a centropelvic recurrence after radical trachelectomy: Literature review and implications for the preoperative selection of patients" (Gynecol Oncol 92:1002-1005) by Morice et al; author reply, Gynecol Oncol 95:414-416, 2004.
19. Bali A, Weekes A, Van Trappen P, et al: Central pelvic recurrence 7 years after radical vaginal trachelectomy. Gynecol Oncol 96:854-856, 2005.
20. Dargent D, Franzosi F, Ansquer Y, et al: Extended trachelectomy relapse: Plea for patient involvement in the medical decision. Bull Cancer 89:1027-1030, 2002.
21. Plante M, Renaud MC, Roy M: Sentinel node evaluation in gynecologic cancer. Oncology 18:75-87, 2002.
22. Wydra D, Sawicki S, Emerich J, et al: Lymphoscintigraphy in radical vaginal trachelectomy and pelvic lymphadenectomy. Nucl Med Rev Cent East Eur 7:187-188, 2004.
23. Singh N, Titmuss E, Aleong JC, et al: A review of post-trachelectomy isthmic and vaginal smear cytology. Cytopathology 15:97-103, 2004.
24. Sahdev A, Jones J, Shepherd JH, et al: MR imaging appearances of the female pelvis after trachelectomy. Radiographics 25:41-52, 2005.
25. Plante M, Renaud MC, Hoskins IA, et al: Vaginal radical trachelectomy: A valuable fertility-preserving option in the management of early-stage cervical cancer. A series of 50 pregnancies and review of the literature. Gynecol Oncol 98:3-10, 2005.
26. Boss EA, van Golde RJ, Beerendonk CC, et al: Pregnancy after radical trachelectomy: A real option? Gynecol Oncol 99(suppl 1):S152-S156,2005.
27. Klemm P, Tozzi R, Kohler C, et al: Does radical trachelectomy influence uterine blood supply? Gynecol Oncol 96:283-286, 2005.
28. Shepherd JH, Mould T, Oram DH: Radical trachelectomy in early stage carcinoma of the cervix: Outcome as judged by recurrence and fertility rates. BJOG 108:882-885, 2001.
29. Kolomainen DF, Herod JJ, Holland N, et al: Actinomyces on a papanicolaou smear following a radical trachelectomy. Br J Obstet Gynaecol 110:1036-1037, 2003.
30. Bernardini M, Barrett J, Seaward G, et al: Pregnancy outcome in patients post radical trachelectomy. Am J Obstet Gynecol 189:1378-1382, 2003.
31. Alexopoulos E, Efkarpidis S, Fay TN, et al: Pregnancy following radical trachelectomy and pelvic lymphadenectomy for stage I cervical adenocarcinoma. Acta Obstet Gynecol Scand 81:791-792, 2002.
32. Lieman JM, Brumfield CG, Carlo W, et al: Preterm premature rupture of membranes: Is there an optimal gestational age for delivery? Obstet Gynecol 105:12-17, 2005.
33. Kenyon S, Boulvain M, Neilson J: Antibiotics for preterm rupture of the membranes: A systematic review. Obstet Gynecol 104:1051-1057, 2004.
34. Iams JD, Goldenberg RL, Meis PJ, et al: The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 334:567-572; 1996.
35. Vintzileos AM: Recurrent pregnancy loss: evaluation and treatment, in Creasy R, Resnik R, Iams J (eds): Maternal-Fetal Medicine Principles and Practices, 5th ed, pp 579-601. Philadelphia, WB Saunders Co, 2004.
36. Creasy R: Preterm labor and delivery, in Creasy R, Resnik R, Iams J (eds): Maternal-Fetal Medicine Principles and Practices, 5th ed, pp 623-661. Philadelphia, WB Saunders Co, 2004.
37. Saling E: Der fruhe muttermundverschluss zur vermeidung habitueller aborte und fruhgeburten. Z Geburtshilfe Perinatol 185:259-261, 1981.
38. Mathevet P, Laszlo de Kaszon E, Dargent D, et al: Fertility preservation in early cervical cancer. Gynecol Obstet Fertil 31:706-712, 2003.
39. Berghella V, Tolosa JE, Kuhlman K, et al: Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol 177:723-730, 1997.
40. Berghella V, Odibo AO, Tolosa JE: Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: A randomized trial. Am J Obstet Gynecol 191:1311-1317, 2004.
41. American College of Obstetricians and Gynecologists: Use of progesterone to reduce preterm birth. ACOG Committee Opinion No. 291. Obstet Gynecol 102:115-1116, 2003.
42. Meis PJ, Klebanoff M, Dombrowski MP, et al: Does progesterone treatment influence risk factors for recurrent preterm delivery? Obstet Gynecol 106:557-561, 2005.
43. Sanchez-Ramos L, Kaunitz AM, Delke I: Progestational agents to prevent preterm birth: A meta-analysis of randomized controlled trials. Obstet Gynecol 105:273-279, 2005.
44. Olsen SF, Secher NJ, Bjornsson S, et al: The potential benefits of using fish oil in relation to preterm labor: The case for a randomized controlled trial? Acta Obstet Gynecol Scand 82:978-982, 2003.
45. Selo-Ojeme DO, Ind T, Shepherd JH: Isthmic stenosis following radical trachelectomy. J Obstet Gynaecol 22:327-328, 2002.
46. Aust TR, Herod JJ, Gazvani R: Placement of a Malecot catheter to enable embryo transfer after radical trachelectomy. Fertil Steril 83:1842, 2005.
47. Smith JR, Boyle DC, Corless DJ, et al: Abdominal radical trachelectomy: A new surgical technique for the conservative management of cervical carcinoma. Br J Obstet Gynaecol 104:1196-1200, 1997.
48. Ungar L, Palfalvi L, Hogg R, et al: Abdominal radical trachelectomy: A fertility-preserving option for women with early cervical cancer. Br J Obstet Gynaecol 112:366-369, 2005.
49. Del Priore G, Ungar L, Smith JR, et al: Regarding "First case of a centropelvic recurrence after radical trachelectomy: Literature review and implications for the preoperative selection of patients," (Gynecol Oncol 92:1002-1005) by Morice et al; letter to the editor, Gynecol Oncol 95:414, 2004.
50. Bader AA, Tamussino KF, Moinfar F, et al: Isolated recurrence at the residual uterine cervix after abdominal radical trachelectomy for early cervical cancer. Gynecol Oncol 99:785-787, 2005.
51. Rodriguez M, Guimares O, Rose PG: Radical abdominal trachelectomy and pelvic lymphadenectomy with uterine conservation and subsequent pregnancy in the treatment of early invasive cervical cancer. Am J Obstet Gynecol 85:370-374, 2001.
52. Abu-Rustum NR, Su W, Levine DA, et al: Pediatric radical abdominal trachelectomy for cervical clear cell carcinoma: a novel surgical approach. Gynecol Oncol 97:296-300, 2005.
53. Dargent D: Radical abdominal trachelectomy and pelvic lymphadenectomy with uterine conservation and subsequent pregnancy in the treatment of early invasive cervical cancer. Am J Obstet Gynecol 187:1728, 2002.
54. Covens A, Rosen B, Murphy J, et al: How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol 84:145-149, 2002.
55. Lee CL, Huang KG, Wang CJ, et al: Laparoscopic radical trachelectomy for stage Ib1 cervical cancer. J Am Assoc Gynecol Laparosc 10:111-115, 2003.
56. Cibula D, Ungar L, Palfalvi L, et al: Laparoscopic abdominal radical trachelectomy. Gynecol Oncol 97:707-709, 2005.
57. Benedetti-Panici P, Greggi S, Colombo A, et al: Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: Results from the Italian multicenter randomized study. J Clin Oncol 20:179-188, 2002.
58. Duenas-Gonzalez A, Cetina L, Mariscal I, et al: Modern management of locally advanced cervical carcinoma. Cancer Treat Rev 29:389-399, 2003.
59. Andrade JM, Marana HR, Mangieri LF, et al: Successful preservation of fertility subsequent to a complete pathologic response of a squamous cell carcinoma of the uterine cervix treated with primary systemic chemotherapy. Gynecol Oncol 77:213-215, 2000.
60. Marana HR, de Andrade JM, da Silva Mathes AC, et al: Chemotherapy in the treatment of locally advanced cervical cancer and pregnancy. Gynecol Oncol 80:272-274, 2001.
61. Tewari K, Cappuccini F, Gambino A, et al: Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: A report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer 82:1529-1534, 1998.
62. Maneo A: Chemo-conization: A more conservative approach. Presented at the Annual Meeting of the International Gynecologic Cancer Society, October 4, 2004; Edinburgh, Scotland.
63. Zanetta G, Fei F, Mangioni C: Chemotherapy with paclitaxel, ifosfamide, and cisplatin for the treatment of squamous cell cervical cancer: The experience of Monza. Semin Oncol 27(suppl 1):23-27, 2000.
64. Plante M, Lau S, Brydon L, et al: Neoadjuvant chemotherapy followd by vaginal radical trachelectomy in bulky stage IB1 cervical cancer. Case report. Gynecol Oncol. In press.
65. Koliopoulos G, Sotiriadis A, Kyrgiou M, et al: Conservative surgical methods for FIGO stage IA2 squamous cervical carcinoma and their role in preserving women's fertility. Gynecol Oncol 93:469-473, 2004.
66. Ueda M, Ueki K, Kanemura M, et al: Conservative excisional laser conization for early invasive cervical cancer. Gynecol Oncol 95:231-234, 2004.
67. Bekkers RL, Keyser KG, Bulten J, et al: The value of loop electrosurgical conization in the treatment of stage IA1 microinvasive carcinoma of the uterine cervix. Int J Gynecol Oncol 12:485-489, 2002.
68. Schorge JO, Lee KR, Sheets EE: Prospective management of stage IA1 cervical adenocarcinoma by conization alone to preserve fertility: A preliminary report. Gynecol Oncol 78:217-220, 2000.
69. Paraskevaidis E, Koliopoulos G, Lolis E, et al: Delivery outcomes following loop electrosurgical excision procedure for microinvasive (FIGO stage IA1) cervical cancer. Gynecol Oncol 86:10-13, 2002.
70. El-Bastawissi AY, Becker TM, Daling JR: Effect of cervical carcinoma in situ and its management on pregnancy outcome. Obstet Gynecol 93:207-212, 1999.
71. Crane JM: Pregnancy outcome after loop electrosurgical excision procedure: A systematic review. Obstet Gynecol 102:1058-1062, 2003.
72. Samson SL, Bentley JR, Fahey TJ, et al: The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol 105:325-332, 2005.
73. Berghella V, Pereira L, Gariepy A, et al: Prior cone biopsy: Prediction of preterm birth by cervical ultrasound. Am J Obstet Gynecol 191:1393-1397, 2004.
74. Argenta PA, Kubicek GJ, Dusenbery KE, et al: Widespread lymph node metastasis in a young women with FIGO stage IA1 squamous cervical carcinoma. Gynecol Oncol 97:659-661, 2005.
75. Nagarsheth N, Maxwell MF, Bentley RC, et al: Bilateral pelvic lymph node metastases in a case of FIGO stage IA1 adenocarcinoma of the cervix. Gynecol Oncol 77:467-470, 2000.
76. Burnett AF, Roman LD, O’Meara AT, et al: Radical vaginal trachelectomy and pelvic lymphadenectomy for preservation of fertility in early cervical carcinoma. Gynecol Oncol 88:419-423, 2003.
77. Schlaerth JB, Spirtos NM, Schlaerth AC: Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer. Am J Obstet Gynecol 188:29-34, 2003.
78. Covens A: Preserving fertility in early stage cervical cancer with radical trachelectomy. Contemp Obstet Gynecol 48:46-66, 2003.
79. Mathevet P, Laszlo de Kaszon E, Dargent D, et al: Fertility preservation in early cervical cancer. Gynecol Obstet Fertil 31:706-712, 2003.