Adjuvant Therapy for Colorectal Cancer: Yesterday, Today, and Tomorrow
During the 1980s, the only drug routinely used to treat colorectal carcinoma was single-agent fluorouracil (5-FU), a drug that had shown no proven benefit in the adjuvant setting. Since then, significant improvements in the overall management of colorectal cancer have been made. This review will compare today's standard of care for adjuvant colorectal carcinoma to that practiced 20 years ago. The authors examine key questions asked about adjuvant therapy and the answers that ultimately changed clinical practice standards and improved overall survival for patients diagnosed with this disease. In addition, this review explores whether 5-FU should be given as part of a multidrug regimen and which route of administration is best when this drug is given. Further, the authors delve into both the use of locally directed therapies to the liver or peritoneum to improve outcomes and the selection of patients to receive adjuvant chemotherapy. Finally, a look to the future shows monoclonal antibodies to be an avenue of great promise in fighting colorectal cancer.
During the 1980s, the only drug routinely used to treat colorectal carcinoma was single-agent fluorouracil (5-FU), a drug that had shown no proven benefit in the adjuvant setting. Since then, significant improvements in the overall management of colorectal cancer have been made. This review will compare today's standard of care for adjuvant colorectal carcinoma to that practiced 20 years ago. The authors examine key questions asked about adjuvant therapy and the answers that ultimately changed clinical practice standards and improved overall survival for patients diagnosed with this disease. In addition, this review explores whether 5-FU should be given as part of a multidrug regimen and which route of administration is best when this drug is given. Further, the authors delve into both the use of locally directed therapies to the liver or peritoneum to improve outcomes and the selection of patients to receive adjuvant chemotherapy. Finally, a look to the future shows monoclonal antibodies to be an avenue of great promise in fighting colorectal cancer.
In reviewing the colon cancer literature from the mid-1980s, a recurrent theme could be summed up as, "The overall picture and results of treatment of colorectal cancer have not changed impressively in the last 20 years."[1] During that era, the only drug routinely used to treat colorectal carcinoma was single-agent fluorouracil (5-FU), a drug that had been in use for many years and had shown no proven benefit in the adjuvant setting.
Fortunately, physicians practicing during those years were dedicated to their search for new, more effective therapies for colorectal cancer, especially in the adjuvant setting. Their efforts have led to a significant improvement in the overall picture and results of colorectal cancer treatment over the past 2 decades. In this review, we will compare today's standard of care for adjuvant colorectal carcinoma to that practiced 20 years ago. In addition, we will examine the key questions asked about adjuvant therapy and the answers that ultimately changed clinical practice standards and improved overall survival for patients diagnosed with this disease.
Even with recent advances, colorectal cancer continues to be the third leading cause of cancer deaths in both men and women, accounting for 10% of all cancer-related deaths annually; in 2005, approximately 56,290 deaths associated with the disease were recorded in the United States.[2] Although these statistics are better than those of 1985, when colorectal cancer caused 12% of all cancer-related deaths,[3] more work needs to be done, and research is ongoing. We close this review by considering the questions being asked in current clinical trials and how they might be answered over the next 20 years.
Role of Adjuvant Therapy in 1985
Adjuvant therapy involves the use of chemotherapy following potentially curative surgical resection to destroy residual micrometastatic disease and to prevent relapse. The theory of adjuvant chemotherapy was initially proposed in the 1950s, but in 1985, doctors were still trying to prove its role in the setting of colorectal cancer therapy.
At that time, single-agent, bolus 5-FU was the mainstay chemotherapeutic agent for metastatic colorectal cancer; however, no benefit in the adjuvant setting had yet been proven. Several randomized clinical trials in the 1970s had compared single-agent 5-FU to observation,[4,5] and results demonstrated a trend toward improved overall survival in the chemotherapy arm, but the improvement was not statistically significant.
The finding of a consistent trend toward longer survival after adjuvant treatment with 5-FU, led investigators to develop strategies to enhance the drug's activity using different delivery methods. Many regimens and modifications have been tested over the past 20 years to address a number of clinically relevant questions. Should 5-FU be given as part of a multidrug regimen? Should 5-FU be administered as a continuous infusion or as an oral formulation? Do locally directed therapies to the liver or peritoneum improve outcomes? And perhaps most importantly, who should receive adjuvant chemotherapy?
Do 5-FU Modulators Increase Efficacy?
In the early 1980s, adjuvant chemotherapy trials began to look at the ability of combination regimens, such as 5-FU plus semustine[6,7] or levamisole,[8] to significantly improve outcomes in the adjuvant setting. These trials demonstrated no statistically significant survival benefit among treated patients compared with those in the observation group, although subgroup analyses of several trials demonstrated a significant improvement in overall survival in patients with stage III disease. The largest benefit for this subgroup was found by Wunderlich and colleagues, who reported a 72% overall survival in the adjuvant chemotherapy arm using a regimen of 5-FU, mitomycin, and cytarabine vs 33% in the observation arm (P< .04).[9] Subsequently, this regimen was abandoned in favor of less toxic approaches.
The results of these subgroup analyses were intriguing, but a planned evaluation of adjuvant therapy in stage III patients was still needed to confirm these findings. A randomized controlled trial comparing observation, levamisole alone, and 5-FU/levamisole in stage III colorectal patients was conducted in the 1980s, and the results were published in 1990.[10] At a median follow-up of 3 years, the population of patients treated with the 5-FU/levamisole combination demonstrated a 41% decrease in risk of recurrence (P< .0001) and, more importantly, a reduction in the death rate by 33% (P= .006). These significant benefits were confirmed at reanalysis after a median follow-up of 6.5 years.[11] Based on these results, a National Cancer Institute consensus panel recommended routine adjuvant use of 5-FU/levamisole in stage III colorectal cancer patients.[12]
5-FU/Leucovorin
Despite the proven benefit of the 5-FU/levamisole combination, investigators continued to look for better regimens, often by testing drugs with proven benefit in metastatic disease in the adjuvant setting. One of the first such combinations to show a benefit was 5-FU/leucovorin. Initial trials were combined in a meta-analysis conducted by the International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators (Table 1).[13-18] For these studies, patients with stage II/III disease were randomized to observation or six cycles of 5-FU/leucovorin.[13] The adjuvant therapy was well tolerated, and its use yielded a 3-year overall survival of 83% vs 78% in the observation-only arm (P= .02). O’Connell and colleagues used a lower leucovorin dose-20 mg/m2 vs the 200 mg/m2 used in trials included in the IMPACT analysis.[19] Even with the lower dose, the treatment was well tolerated and the 5-FU/leucovorin regimen continued to demonstrate a survival benefit compared with observation.
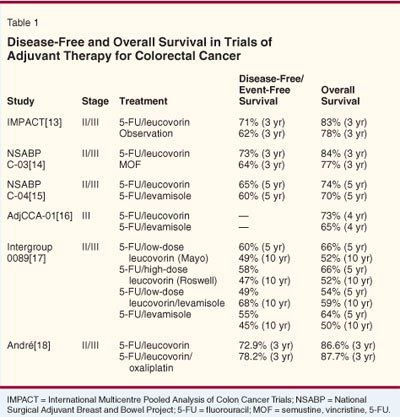
The National Surgical Adjuvant Breast and Bowel Project (NSABP C-03) took the next step and compared 5-FU/leucovorin with MOF (semustine [methyl-CCNU], vincristine [Oncovin], and 5-FU) in 1,081 stage II /III colorectal cancer patients. The data from that trial showed the leucovorin arm to have a 3-year overall survival rate of 84%, compared with 77% in the MOF group (P= .03).[14]
Leucovorin vs Levamisole
The NSABP then compared levamisole to leucovorin in 2,151 stage II/III colorectal cancer patients who received either 5-FU/leucovorin, 5-FU/levamisole, or a combination of all three drugs. [15] When compared with those in the 5-FU/levamisole arm, the patients who received 5-FU/leucovorin had a longer 5-year disease-free survival rate (65% vs 60%, P= .04) but no significant overall survival benefit (overall survival: 74% vs 70%; P= .07). The three-drug combination offered no additional benefit over the two-drug regimen.
Another trial of 680 patients also demonstrated the superiority of leucovorin over levamisole in patients with stage III disease.[16] After a median follow-up of 46.5 months, the 5-FU/leucovorin arm had a lower mortality rate (P= .0089) and a 4-year overall survival of 63 months, compared with 55 months in the 5-FU/levamisole arm. These results, published in 2001, led to the adoption of 5-FU/leucovorin as the standard of care in the adjuvant setting and in future adjuvant trials.
In December 2005, publication of 10-year Intergroup 0089 follow-up data brought another important update to the leucovorin-vs-levamisole debate.[17] In this trial, 3,794 patients with stage II/III colon cancer were randomized to 1 year of levamisole plus 5-FU or to one of three experimental treatment regimens: low-dose leucovorin/5-FU (Mayo Clinic regimen) given over 6 to 8 months, high-dose leucovorin/5-FU (Roswell Park regimen) given over 8 months, or a low dose of leucovorin/5-FU plus levamisole given for 6 months. No difference in overall survival was noted among the four arms, with overall survival at 10 years ranging from 50% to 59%. However, the leucovorin toxicity profile, especially at the higher dose, was superior to levamisole, with more grade 3/4 diarrhea but less neutropenia and stomatitis recorded. Additionally, the superiority of leucovorin was reaffirmed, as investigators reported that leucovorin given for 6 to 8 months was as effective as levamisole given for 12 months.
The high- and low-dose leucovorin regimens are both considered acceptable alternatives. However, the high-dose Roswell Park regimen is more frequently employed by oncologists using 5-FU/leucovorin, because it is associated with less neutropenia and alopecia.
How Should Systemic 5-FU Be Administered?
When it first came into use, 5-FU was delivered as a bolus injection. However, because the drug is metabolized rapidly and has a short serum half-life (< 20 minutes), researchers became interested in the potential for increasing its benefits when administering it as a continuous infusion rather than as a bolus injection.
In the 1970s, Seifert et al reported that continuous-infusion 5-FU in advanced-stage colorectal cancer yielded an increased response rate (44%) over bolus injections (22%).[20] The two routes also showed a different side-effect profile, with myelotoxicity more severe in the bolus arm-31% of patients reached a total white blood cell count (WBC) of less than 2,000 cells/mm³, whereas no patients in the continuous-infusion arm had a WBC that low. The improved response rate found with continuous-infusion 5-FU in the metastatic setting led to several trials of the regimen in the adjuvant setting.
In 2003, Saini et al reported on a randomized control trial of 716 patients with stage II/III disease who were given either six monthly bolus injections of 5-FU/leucovorin (425 mg/m2 of 5-FU, 20 mg/m2 of leucovorin on days 1-5) or continuous-infusion 5âFU (300 mg/m2/d for 12 weeks).[21] At a median follow-up of almost 20 months, no difference in overall survival was noted. However, more patients in the bolus-injection arm than in the continuous-infusion arm were alive and free of relapse at follow-up (80% vs 68.6%, P = .023). The continuous-infusion arm also had an improved quality-of-life score and significantly fewer episodes of grade 3/4 neutropenia, alopecia, diarrhea, and stomatitis (P< .0001).
The decrease in toxicity seen with continuous-infusion 5-FU was supported by subsequent trials. Intergroup 0153 compared bolus 5-FU/leucovorin/levamisole for six cycles to continuous-infusion 5-FU/levamisole given in three 8-week cycles.[22] The bolus regimen consisted of the Mayo Clinic regimen of 425 mg/m2 of 5-FU and 20 mg/m2 of leucovorin given for 5 days every 4 to 5 weeks, and the continuous-infusion regimen was 250 mg/m2/d of 5-FU given for three cycles of 56 days. As with previous trials, the most significant difference between the two regimens was the toxicity profile. In the continuous-infusion arm, only 5% of patients experienced a grade 4 toxicity, compared with 39% of patients in the bolus-infusion arm.
Despite the overall decreased toxicity, patients in the continuous-infusion arm were almost twice as likely to withdraw from the protocol before completion. Early discontinuation seemed most related to inconvenience of the pump, chronic hand-foot syndrome, and clotting episodes, all of which were more common in the infusional arm. Unlike Saini's results, this trial demonstrated no significant difference in disease-free survival; like the Saini study, it showed no difference in overall survival between the two arms.
Why the Differences in Response and Toxicity?
As research into continuous infusion vs bolus injections of 5-FU progressed, the understanding of its mechanism of action matured. After analysis of the clinical and preclinical data, Sobrero and colleagues proposed that 5-FU had a different mechanism of action depending upon the duration of administration.[23] Bolus 5-FU injection more likely affected DNA replication, whereas infusion of the drug more likely influenced RNA transcription.
This theory was well supported by the differences in toxicity profile and mechanism of resistance seen between infusional and bolus regimens. Clinical data showed that some patients responded to infusional 5-FU even after their disease progressed during use of 5-FU given via bolus injection, and this supported Sobrero's theory that the duration of 5-FU administration led to differences in the drug's mechanism of action. Based on his analysis, Sobrero proposed that a combination regimen of bolus and infusional 5-FU may yield higher clinical response rates than either regimen alone.
To examine this theory, de Gramont and colleagues created a clinical trial comparing bolus 5-FU injection to a combined bolus-injection/continuous-infusion 5-FU regimen.[24] In this trial, 905 patients were randomized to the infusional arm (200 mg/m2 of leucovorin followed by 400 mg/m2 of 5-FU given via a bolus injection, followed by a 22-hour continuous infusion of 600 mg/m2 of 5-FU repeated every 2 weeks for 6 or 9 months) or to the control arm (200 mg/m2 of leucovorin plus 400 mg/m2 of 5-FU via bolus injection for 5 days each month for 6 or 9 months). As with previous trials, the patients enrolled on the infusional treatment arm had fewer grade 3/4 toxicities (P< .001). However, combining 5-FU via bolus injection and continuous infusion did not improve outcomes-a similar 3-year overall survival not affected by duration of therapy was noted in both groups.
No clear survival benefit to using infusional 5-FU rather than bolus 5-FU in the adjuvant setting has been found. However, the improved toxicity profile demonstrated in several trials has led many oncologists to use infusional regimens in the adjuvant setting when there is a reason not to use today's standard of care-ie, a regimen of oxaliplatin (Eloxatin) plus 5-FU.
Can 5-FU Be Given Orally?
Most patients would prefer a pill to intravenous (IV) chemotherapy, and investigators have tried to develop an effective oral fluoropyrimidine. Because of its erratic absorption profile, 5-FU is not a suitable agent for oral administration. Therefore, prodrugs or combinations of 5-FU with agents that promote reliable absorption have been developed.
Japanese investigators have been particularly interested in the use of oral preparations. In 2004, a Japanese group published an analysis of three randomized, controlled trials comparing adjuvant use of oral fluorinated pyrimidines with observation. The analysis included 585 patients with stage I colorectal cancer, 2,295 with stage II disease, and 2,348 with stage III disease.[25] The combined results of these trials demonstrated a statistically significant improved overall survival in the chemotherapy arms compared to the observation arm, with a hazard ratio (HR) of 0.89. Further subgroup analysis confirmed this benefit in all stages of disease.
However, before oral fluorinated pyrimidines could be used routinely, they needed to be compared with an IV formulation. In 2005, Cassidy and colleagues tested the oral fluorinated pyrimidine capecitabine (Xeloda), at 1,250 mg/m2 bid on days 1-14 of a 21-day cycle, with bolus 5-FU/leucovorin (20 mg/m2 of leucovorin plus 425 mg/m2 of 5-FU on days 1-5 of a 28-day cycle).[26] The two regimens were at least equivalent with regard to disease-free and overall survival. The disease-free survival hazard ratio of 0.87 (range: 0.75-1.00), however, suggested that the oral regimen might have superior efficacy. The incidence of grade 3/4 toxicities was significantly lower in the capecitabine group (P< .001), with less neutropenia (2% vs 26%) but more hand-foot syndrome (17% vs 1%) and asymptomatic hyperbilirubinemia (20% vs 6%).
This trial led to the approval of capecitabine in the United States as an alternative to IV 5-FU in the adjuvant setting. Ongoing trials are evaluating the capecitabine/oxaliplatin combination, with early safety results demonstrating grade 3/4 toxicity rates similar to those seen in bolus 5-FU/leucovorin regimens.[27] The oxaliplatin/capecitabine regimen now is being compared to FOLFOX4 (5-FU/leucovorin plus oxaliplatin) alone and with bevacizumab (Avastin) in the AVANT adjuvant trial.
Do Locally Directed Therapies Improve Outcomes?
After a potentially curative surgery, recurrence is frequently detected in the surgical bed, peritoneal space, or liver. This relapse pattern led physicians to investigate locally directed therapies, such as 5-FU given intra-peritoneally (IP) or infused directly into the hepatic vasculature.
In 1985, Sugarbaker et al published the results of a trial comparing IV vs IP 5-FU in patients with high-risk stage II/III colorectal cancer.[28] Although there was a decreased incidence of peritoneal carcinomatosis in the IP group, there was no difference in distant metastases, time to relapse, or overall survival rate. Subsequent trials used bimodality therapy with IP infusions plus IV 5-FU/leucovorin.[29-31] The chemotherapy group showed an overall survival of 70%, whereas the observation group showed an overall survival of 63% (P= .05).[29] In later trials, when bimodality treatment was compared to IV therapy alone, there was a decreased incidence of locoregional recurrence (P= .0005) but no survival benefit when IP therapy was added in the adjuvant setting.[30,31] Thus, studies of the addition of IP therapy have yet to prove a clear benefit of this approach over IV or oral therapy, and IP therapy has not been incorporated in standard adjuvant chemotherapy regimens.
Liver Metastases
The liver is the most frequent site of metastatic disease in colon cancer, and direct infusion of chemotherapy into the liver has been of long-time interest. In 1985, the first trial demonstrating a benefit from direct liver prophylaxis was published.[32] Investigators randomly assigned 244 patients with stage I-III disease to observation or a 7-day portal vein infusion of 5-FU plus heparin. As predicted, the incidence of hepatic metastases was decreased in the intervention group, with 5 patients developing liver disease vs 22 in the control group. The study also revealed an overall survival benefit (P= .002) and a minimal difference in the morbidity experienced by the two groups.
The NSABP conducted a larger trial of portal-vein 5-FU/heparin infusion vs observation alone (NSABP C-02), in 1,158 patients with stage I-III disease.[33] Their results also suggested a benefit to therapy: After a median follow-up of 41.8 months, they found a trend toward improved survival in the 5-FU arm (81% vs 73%; P = .07) and a significant improvement in disease-free survival (74% vs 64%, P = .02). However, there was no decrease in hepatic metastases, which led the authors to hypothesize that any survival benefit was due to the systemic effects of the 5-FU rather than to the local effects on micrometastatic disease in the liver.
The hepatic artery provides most of the blood supply to liver metastases, which led investigators to direct chemotherapy infusion via the hepatic artery in the adjuvant setting. Sadahiro and colleagues randomized 316 patients with stage II/III colon cancer to postoperative hepatic artery infusion of 5-FU vs surgery alone.[34] After a median follow-up of 59 months, a decreased incidence of metastasis to the liver, but not to other sites, was recorded in the infusion arm. Overall survival was also superior (P= .0005) in infusion recipients at 3 years (94% vs 82%) and at 5 years (89% vs 76%). Because no data comparing hepatic intra-arterial infusion to systemic therapy in the adjuvant setting are available and delivery of chemotherapy into the hepatic artery is so difficult, the usefulness of this approach is limited, and it is not currently part of the standard treatment regimen.
Local vs Systemic Therapy
Initial trials of locally directed therapy compared this strategy to observation. By the early 1990s, however, adjuvant IV chemotherapy was the standard of care in patients with stage III disease, and a definitive trial was still needed to prove whether locally directed therapies were superior to IV chemotherapy. In 2004, Labianca and colleagues randomized 1,084 patients to adjuvant therapy with intraportal infusions, IV 5-FU/leu-covorin, or a combined regimen.[35] They found a similar disease-free and overall survival in all three arms. Nordlinger et al conducted a similar trial of systemic 5-FU plus leucovorin or levamisole, compared to IV plus regional therapy (415 patients received intraperitoneal therapy, and 235 received intraportal therapy).[36] The 5-year overall survival of 72% was unchanged when locoregional therapy was added to the IV regimen.
Most medical oncologists are more comfortable with IV chemotherapy, and this route continues to be the standard of care. With the proven benefit of adding oxaliplatin to the 5-FU regimen, IV therapy is even more clearly the preferred regimen.
Should 5-FU Be Part of a Multidrug Regimen?
Addition of Oxaliplatin
After the benefits of oxaliplatin were demonstrated in the metastatic setting, André and colleagues tested it in the adjuvant setting.[18] This trial included 2,246 stage II/III colon cancer patients undergoing adjuvant chemotherapy with 5-FU/leucovorin with or without oxaliplatin. The probability of disease-free survival at 3 years was 78.2% in the oxaliplatin group vs 72.9% in the control group (P= .002). A relative risk reduction of 24% was still present when the data were reanalyzed in 2005 after a median follow-up of 48.6 months.[37] Thus far, no overall survival benefit has been demonstrated from the addition of oxaliplatin, but the median 38-month follow-up at the last analysis was relatively short; 5-year overall survival data should be available in the next year.
With the exception of neuropathy, the addition of oxaliplatin to 5-FU/leucovorin did not significantly change the toxicity profile. However, 92% of patients receiving oxaliplatin had some neurosensory symptoms, and grade 3 sensory neuropathy, indicating interference with daily activities, was observed in 12.4% of patients.[18] After 4 years of follow-up, 3% of patients treated on the oxaliplatin arm still had moderate-to-severe paresthesias.[37]
The NSABP C-07 trial confirmed the benefit of oxaliplatin in the adjuvant setting.[38] The addition of oxaliplatin to weekly 5-FU/leucovorin increased 3-year disease-free survival from 71.6% to 76.5% (P = .004). As with previous trials, the main oxaliplatin toxicity was neurosensory, with grade 3 neuropathy occurring in 8% of treated patients and 1% of controls. The decrease in the incidence of severe neurotoxicity correlated with the lower cumulative oxaliplatin dose in the bolus 5-FU-based FLOX regimen (leucovorin [folinic acid]/oxaliplatin) of the NSABP trial, compared with the infused 5-FU-based FOLFOX regimen used in the MOSAIC trial (Multicenter International Study of Oxaliplatin/5âFU/Leucovorin in the Adjuvant Treatment of Colon Cancer). Despite increased neuropathy, the improved outcomes seen with the oxaliplatin/5-FU/ leucovorin combination led to its adoption as the standard of care in the adjuvant setting.
Addition of Irinotecan
Due to its benefit against metastatic disease,[39] irinotecan (Camptosar) has also been evaluated as adjuvant therapy. Unlike oxaliplatin, irinotecan did not improve disease-free survival in this setting in three recently presented studies. The PETACC 3 (Pan European Trial in Adjuvant Colon Cancer) trial randomized 3,278 patients with stage II/III disease to 5âFU/leucovorin with or without irinotecan.[40] The planned primary end point of the trial was efficacy in patients with stage III disease; this group showed no statistically significant improvement in 3-year disease-free survival when compared to patients who were not given irinotecan (62.9% vs 59.9%, respectively). Analysis of the combined results for stage II/III patients subsequently demonstrated an improved disease-free survival in the combined group (HR = 0.87, P= .038). However, this was not a primary objective of this trial, and the benefit was not seen in other adjuvant trials.
The French ACCORD02 trial randomized 400 patients with high-risk, stage III disease based on perforation, obstruction, and the presence of multiple involved lymph nodes to adjuvant 5-FU/leucovorin therapy with or without irinotecan.[41] Similar to the PETACC3 results, outcomes in this trial showed no difference in event-free survival when irinotecan was added. However, the irinotecan arm had significantly more patients with negative prognostic indicators such as T3/T4 disease, more than 15 involved lymph nodes, and vascular invasion-all of which may have skewed the results against the irinotecan arm.
Although results of the ACCORD02 and the PETACC3 trials both showed increased toxicity (especially grade 3/4 neutropenia) in the irinotecan arm, neither showed increased mortality. This, however, was not the case for the CALGB's adjuvant irinotecan trial,[42] which was stopped early by the Data Safety Monitoring Committee when a 2.8% death rate was detected during treatment with irinotecan as compared with 1.0% in the 5-FU/leucovorin arm (P= .008). At this time, irinotecan is not recommended in the adjuvant setting because of concerns about increased toxicity and the lack of proven benefit.
Which Patients Should Get Adjuvant Therapy?
Although the early chemotherapy trials showed no improvement in overall survival with adjuvant therapy, subgroup analyses frequently demonstrated a benefit for stage III patients who received chemotherapy. Subsequent trials confirmed this benefit,[43] and adjuvant chemotherapy is the standard of care for patients with stage III disease. The benefit of chemotherapy in stage II disease is not as clear, partly because smaller numbers of patients are enrolled in such trials.
In 1999, the Journal of Clinical Oncology published two large analyses of combined stage II data from multiple adjuvant trials.[44,45] Interestingly, the authors of the two analyses came to opposite conclusions despite similar results. In the first analysis, the data from NSABP C-01, C-02, C-03, and C-04 trials were combined and analyzed.[44] The cumulative data demonstrated a 30% reduction in the risk of death at 5 years in groups receiving the superior adjuvant therapy of each trial. On a percentage basis, this benefit was more pronounced than in stage III patients, who showed an 18% reduction in mortality with treatment.
The IMPACT B2 investigators also analyzed a number of adjuvant trials.[45] After studying the results from 1,016 stage II patients treated with 5-FU/leucovorin or observation, this group found no difference in 5-year overall survival rates (82% in treated patients vs 80% in controls).
In 2002, there was still no good answer about whether adjuvant therapy affects survival in stage II colorectal cancer patients. Schrag's team conducted a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) Medicare database to compare outcomes in older patients (aged 65 to 75 years) who were diagnosed with stage II disease and who did or did not receive adjuvant chemotherapy.[46] In this population-based review, 27% of patients were treated with adjuvant chemotherapy. Those more likely to be treated were younger, Caucasian, and otherwise healthy or had high-grade tumors. The 5-year survival data for the two groups were similar: 75% for those who received no chemotherapy vs 78% for treated patients.
More recently, Gill and colleagues published a pooled analysis of over 3,300 patients with stage II/III colon cancer who were randomized to no treatment or adjuvant 5-FU-based chemotherapy.[47] A combined analysis showed that adjuvant chemotherapy produced a 5-year overall survival of 71%, compared with 64% for those given surgery alone. Univariate analyses of prognostic subsets (eg, T stage, grade, age, sex, location of tumor, and nodal status) showed a statistically significant survival benefit from adjuvant therapy among patients with stage III disease as well as among stage II/III patients who had a high tumor grade and higher T stage. Using these findings, an Internet program (available at www.mayoclinic.com/calcs) was created to help providers determine an individual patient's possible benefit from adjuvant chemotherapy.
In 2004, the American Society of Clinical Oncology published guidelines on the use of adjuvant chemotherapy in stage II colon cancer.[48] After conducting a meta-analysis of studies published through May 2003, the expert panel found no statistically significant survival benefit from the use of adjuvant chemotherapy in stage II disease. However, after considering the clear benefit demonstrated in stage III disease, the reviewers concluded that adjuvant treatment could reasonably be offered to patients with stage II, high-risk disease; this would include individuals with T4 lesions, poorly differentiated histology, insufficient lymph node sampling (< 12 nodes), or bowel perforation.
Guidelines and meta-analyses aside, the final decision about using chemotherapy should be made on a case-by-case basis after extensive discussion between patient and physician.
What Questions Are Physicians Asking Today?
The goal of physicians over the next 20 years will be to improve adjuvant therapy by maximizing benefits and minimizing toxicities. One way to achieve this is by further defining subgroups of patients who benefit most from adjuvant therapy, thus allowing treatment decisions to be better tailored to individual patients. Another method is to develop less toxic therapies, such as the use of monoclonal antibodies.
Molecular Markers
The benefit and use of adjuvant therapy in stage II disease is still unclear. However, several molecular markers (ie, microsatellite instability [MSI], loss of heterozygosity of the 18q allele, and DNA ploidy) appear to correlate with clinical outcomes and may help direct therapy in the future. In a pooled analysis of 32 trials including 7,642 patients with colorectal cancer, 1,277 of whom had MSI, patients with high instability had better outcomes, with a hazard ratio for overall survival of 0.65.[49] In fact, a subanalysis of a small group of patients from two of these trials found no benefit from adjuvant 5-FU-based chemotherapy in patients with MSI (HR = 1.24). In patients with microsatellite-stable tumors, however, the hazard ratio was 0.72 (P= .007).
Loss of heterozygosity of allele 18q has been associated with inferior outcomes as well. Watanabe and colleagues’ analysis of molecular predictors of survival in stage III and high-risk stage II colon cancer found a 5-year overall survival rate of 69% in patients with retention of 18q alleles vs only 50% in patients who were 18q-negative.[50]
The presence of nondiploid DNA has also been suggested to be a predictor of inferior outcomes in stage II/III colon cancer. Multivariate analysis of pooled data on 528 patients demonstrated that DNA ploidy was an independent prognostic variable of outcome, with an overall survival hazard ratio of 0.62 for patients with diploid tumors.[51] Unlike the preceding trials, neither MSI nor 18q status were found to be independent predictors of outcome in this study.
The disparate outcomes and retrospective nature of these analyses have not given us a true evaluation of the prognostic nature of any of these markers. Prospectively gathered data must be obtained before oncologists can make treatment decisions based on any of these molecular markers. Although not a comparative trial, Eastern Cooperative Oncology Group (ECOG) 5202 will provide additional information on this topic. This phase III trial will compare 5-FU/leucovorin/oxaliplatin with or without bevacizu-mab in patients with high-risk stage II disease; inclusion criteria for high risk are loss of heterozygosity of 18q plus microsatellite stability. The results of ECOG 5202 and subsequent trials should help to answer questions concerning molecular markers and adjuvant therapy for colorectal cancer.
Monoclonal Antibodies
Monoclonal antibodies have demonstrated improved outcomes and decreased toxicity in several malignancies, including metastatic colon cancer. In the adjuvant setting, cetuximab (Erbitux) and bevacizumab are two antibodies of particular interest because of their proven benefit against metastatic disease.
Cetuximab binds to and inhibits the epidermal growth factor receptor, which is overexpressed in 60% to 80% of colorectal cancers and is associated with a shorter survival. This agent has shown clinical activity in metastatic disease, both as monotherapy and in combination with irinotecan and FOLFOX4.[52,53] Bevacizumab, a vascular endothelial growth factor inhibitor, has also improved survival when added to regimens that include irinotecan, 5-FU/leucovorin, or oxaliplatin.[54,55] Trials of both of these drugs coupled with FOLFOX chemotherapy are currently accruing patients with stage III disease in the adjuvant setting.
Conclusions
Although the outlook for patients with stage II/ III colorectal cancer is far better than it was 20 years ago, improvements are needed until all patients can be cured. Over 2 decades, the state of the science has improved from a point at which half of patients with stage III disease were cured to a time when cure is achieved in three-quarters of such patients.
A host of new agents for colorectal cancer, both on the horizon and on the market, may be useful in the adjuvant setting. Presently, bevacizumab and cetuximab are being evaluated with chemotherapy. In the future, the prospect of individualized medicine will likely become a practical reality. With the submission of simple blood samples analyzed for specific pharmacogenetic profiles, we hope to pinpoint populations of patients who will benefit most from specific chemotherapeutic agents or combinations and who will be less susceptible to significant toxicities. In addition, many new agents in clinical development will undoubtedly have a positive impact on prognosis for patients diagnosed with stage II/III colorectal cancer in the year 2026.
References:
1. Metzger UF, Ghosh BC, Kisner DL: Adjuvant treatment of colorectal cancer: Current status and concepts. Cancer Chemother Pharmacol 14:1-8, 1985.
2. Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005.
3. Silverberg E, Lubera JA: Cancer statistics, 1988. CA Cancer J Clin 38:5-22, 1988.
4. Grage TD, Metter GE, Cornell GN, et al: Adjuvant chemotherapy with 5-fluorouracil after surgical resection of colorectal carcinoma (COG protocol 7041). A preliminary report. Am J Surg 133:59-66, 1977.
5. Higgins GA, Humphrey E, Juler G, et al: Adjuvant chemotherapy in the surgical treatment of large bowel cancer. Cancer 38:1461-1467, 1976.
6. Higgins GA, Amadeo JH, McElhinney J, et al: Efficacy of prolonged intermittent therapy with combined 5-fluorouracil and methyl-CCNU following resection for carcinoma of the large bowel. A Veterans Administration Surgical Oncology Group report. Cancer 53:1-8, 1984.
7. Adjuvant therapy of colon cancer-results of a prospectively randomized trial. Gastrointestinal Tumor Study Group. N Engl J Med 10:737-743, 1984.
8. Laurie JA, Moertel CG, Fleming TR, et al: Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol 7:1447-1456,1989.
9. Wunderlich M, Schiessel R, Rainer H, et al: Effect of adjuvant chemo- or immunotherapy on the prognosis of colorectal cancer operated for cure. Br J Surg 72(suppl):S107-S110, 1985.
10. Moertel CG, Fleming TR, Macdonald JS, et al: Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352-358, 1990.
11. Moertel CG, Fleming TR, Macdonald JS, et al: Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med 122:321-326, 1995.
12. NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 264:1444-1450, 1990.
13. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 345:939-944, 1995.
14. Wolmark N, Rockette H, Fisher B, et al: The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 11:1879-1887, 1993.
15. Wolmark N, Rockette H, Mamounas E, et al: Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 17:3553-3559, 1999.
16. Porschen R, Bermann A, Loffler T, et al: Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage IIII colon cancer: Results of the trial adjCCA-01. J Clin Oncol 19:1787-1794, 2001.
17. Haller DG, Catalano PJ, Macdonald JS, et al: Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of intergoup 0089. J Clin Oncol 23:8671-8678, 2005.
18. André T, Boni C, Mounedji-Boudiaf L, et al: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343-2351, 2004.
19. O’Connell MJ, Mailliard JA, Kahn MJ, et al: Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 15:246-250, 1997.
20. Seifert P, Baker LH, Reed ML, et al: Comparison of continuously infused 5-fluorouracil with bolus injection in treatment of patients with colorectal adenocarcinoma. Cancer 36:123-128, 1975.
21. Saini A, Norman AR, Cunningham D, et al: Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer 88:1859-1865, 2003.
22. Poplin EA, Benedetti JK, Estes NC, et al: Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol 23:1819-1825, 2005.
23. Sobrero AF, Aschele C, Bertino JR: Fluorouracil in colorectal cancer-a tale of two drugs: Implications for biochemical modulation. J Clin Oncol 15:368-381, 1997.
24. André T, Colin P, Louvet C, et al: Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: Results of a randomized trial. J Clin Oncol 21:2896-2903, 2003.
25. Sakamoto J, Ohashi Y, Hamada C, et al: Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. Meta-analysis Group of the Japanese Society for Cancer of the Colon and Rectum, and the Meta-Analysis Group in Cancer. J Clin Oncol 22:484-492, 2004.
26. Twelves C, Wong A, Nowacki MP, et al: Capecitabine as adjuvant treatment for stage III colon cancer. N Eng J Med 352:2696-3704, 2005.
27. Schmoll HJ, Tabernero J, Nowacki M, et al: Early safety findings from a phase III trial of capecitabine plus oxaliplatin (XELOX) vs. bolus 5-FU/LV as adjuvant therapy for patients (pts) with stage III colon cancer (abstract 3523). J Clin Oncol 23(16S):251s, 2005.
28. Sugarbaker PH, Gianola FJ, Speyer JC, et al: Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 98:414-422, 1985.
29. Scheithauer W, Kornek G, Rosen H, et al: Combined intraperitoneal plus intravenous chemotherapy after curative resection for colonic adenocarcinoma. Eur J Cancer 31A:1981-1986, 1995.
30. Scheithauer W, Kornek GV, Marczell A, et al: Combined intravenous and intraperitoneal chemotherapy with fluorouracil + leucovorin vs. fluorouracil + levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer 77:1349-1354, 1998.
31. Vaillant JC, Nordlinger B, Deuffic S, et al: Adjuvant intraperitoneal 5-fluorouraceil in high-risk colon cancer: A multicenter phase III trial. Ann Surg 231:449-456, 2000.
32. Taylor I, Machin D, Mullee M, et al: A randomized controlled trial of adjuvant portal vein cytotoxic perfusion in colorectal cancer. Br J Surg 72:359-363, 1985.
33. Wolmark N, Rockette H, Wickerham DL, et al: Adjuvant therapy of Dukes’ A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: Preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C-02. J Clin Oncol 8:1466-1475, 1990.
34. Sadahiro S, Suzuki T, Ishikawa K, et al: Prophylactic hepatic arterial infusion chemotherapy for the prevention of liver metastasis in patients with colon carcinoma. Cancer 100:590-597, 2004.
35. Labianca R, Fossati R, Zaniboni A, et al: Randomized trial of intraportal and/or systemic adjuvant chemotherapy in patients with colon carcinoma. J Natl Cancer Inst 96:750-758, 2004.
36. Nordlinger B, Rougier P, Arnaud JP, et al: Adjuvant regional chemotherapy and systemic chemotherapy versus systemic chemotherapy alone in patients with stage II-III colorectal cancer: A multicentre randomized controlled phase III trial. Lancet Oncol 6:459-468, 2005.
37. de Gramont A, Boni C, Navarro M, et al: Oxaliplatin/5FU/LV in the adjuvant treatment of stage II and stage III colon cancer: Efficacy results with a median follow-up of 4 years (abstract 3501). J Clin Oncol 23(16S):246s, 2005.
38. Wolmark N, Wieand S, Kuebler JP, et al: A phase III trial comparing FULV to FULV + oxaliplatin in stage II or III carcinoma of the colon: Results of NSABP protocol C-07 (abstract LBA3500). J Clin Oncol 23(16S):246s, 2005.
39. Goldberg RM, Sargent DJ, Morton RF, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23-30, 2004.
40. Van Cutsem E, Labianca R, Hossfeld D, et al: Randomized phase III trial comparing infused irinotecan/5-fluorouracil (5-FU)/folinic acid (IF) versus 5-FU/FA (F) in stage III colon cancer patients. (PETACC 3; V307)(abstract LBA8). J Clin Oncol 23(16S):3s, 2005.
41. Ychou M, Raoul JL, Douillard JY, et al: A phase III randomized trial of LV5FU2+CPT-11 vs. LV5FU2 alone in adjuvant high risk colon cancer (FNCLCC Accord02 FFCD9802)(abstract 3502). J Clin Oncol 23(16S):246s, 2005.
42. Saltz LB, Niedzwiecki D, Hollis D, et al: Irinotecan plus fluorouracil/leucovorin (IFL) versus fluorouracil/leucovorin alone (FL) in stage III colon cancer (intergroup trial CALGB C89803)(abstract 3500). Proc Am Soc Clin Oncol 22:245s, 2004.
43. Moertel CG, Fleming TR, Macdonald JS, et al: Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med 122:321-326, 1995.
44. Mamounas E, Wieand S, Wolmark N, et al: Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: Results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04). J Clin Oncol 17:1349-1355, 1999.
45. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) investigators. J Clin Oncol 17:1356-1363, 1999.
46. Schrag D, Rifas-Shiman S, Saltz L, et al: Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol 20:3999-4005, 2002.
47. Gill S, Loprinzi CL, Sargent DJ, et al: Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 22:1797-1806, 2004.
48. Benson AB, Schrag D, Somerfield MR, et al: American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 22:3408-3419, 2004.
49. Popat S, Hubner R, Houlston RS: Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23:609-618, 2005.
50. Watanabe T, Wu TT, Catalano PJ, et al: Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Eng J Med 344:1196-1206, 2001.
51. Sinicrope FA, Halling KC, French A, et al: DNA ploidy is a stronger prognostic variable compared to microsatellite instability or 18q allelic loss in patients with stages II and III colon cancer (abstract 9523). J Clin Oncol 23(16S):842s, 2005.
52. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
53. Tabernero JM, Van Cutsem E, Sastre J, et al: An international phase II study of cetuximab in combination with oxaliplatin/5-fluorouracil (5-FU)/folinic acid (FA) (FOLFOX-4) in the first-line treatment of patients with metastatic colorectal cancer (CRC) expressing epidermal growth factor receptor (EGFR). Preliminary results (abstract 3512). Proc Am Soc Clin Oncol 23:248, 2004.
54. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Eng J Med 350:2335-2342, 2004.
55 Kabbinavar FF, Schulz J, McCleod M, et al: Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol 23:3697-3705, 2005.