Follicular Lymphoma: Expanding Therapeutic Options
The most common indolent lymphoma, follicular lymphoma comprises 35% of adult non-Hodgkin’s lymphoma (NHL) in the United States and 22% worldwide. Features associated with adverse outcome include age, male gender, disease stage, and performance status, with the International Prognostic Index being the most widely used risk classification system. Long-term disease-free survival is possible in select patient subgroups after treatment, but very late relapses suggest that quiescent lymphoma cells might be harbored for long periods of time. Radiation therapy is the mainstay of treatment for limited-stage follicular lymphoma, but there is some experience with chemotherapy and combined chemoradiation. When to initiate treatment in patients with advanced disease is controversial, but options include various combined chemotherapy regimens, monoclonal antibodies, radiolabeled antibodies, and bone marrow or stem cell transplantation. Future directions in the treatment of follicular lymphoma include vaccines, antisense therapy, and proteasome inhibitors.
The most common indolent lymphoma, follicular lymphoma comprises 35% of adult non-Hodgkin’s lymphoma (NHL) in the United States and 22% worldwide. Features associated with adverse outcome include age, male gender, disease stage, and performance status, with the International Prognostic Index being the most widely used risk classification system. Long-term disease-free survival is possible in select patient subgroups after treatment, but very late relapses suggest that quiescent lymphoma cells might be harbored for long periods of time. Radiation therapy is the mainstay of treatment for limited-stage follicular lymphoma, but there is some experience with chemotherapy and combined chemoradiation. When to initiate treatment in patients with advanced disease is controversial, but options include various combined chemotherapy regimens, monoclonal antibodies, radiolabeled antibodies, and bone marrow or stem cell transplantation. Future directions in the treatment of follicular lymphoma include vaccines, antisense therapy, and proteasome inhibitors.
Follicular lymphoma is a type of non-Hodgkin's lymphoma (NHL) that arises from the follicle center B lymphocytes and has a follicular pattern on histopathologic examination.[1] It is the most common indolent lymphoma, comprising 35% of adult NHL in the United States and 22% worldwide.[2] It most commonly is seen in middle-aged individuals and the elderly, with a median age of diagnosis of 59 years, as compared to 64 years for patients with diffuse large B-cell lymphoma. Females (52%) are slightly more often affected than males (48%).[3,4] Morphologically, follicular lymphoma is composed of a mixture of centrocytes (cleaved follicle center cells) and centroblasts (large noncleaved follicle center cells), with centrocytes being the predominant cell type and centroblasts being in the minority.[ 5] The most reproducible method of grading follicular lymphoma, as described by Berard and Mann, is based on the absolute number of centroblasts in 10 neoplastic follicles, expressed per 40* high power field (hpf): grade 1 is defined as 0-5 centroblasts/ hpf; grade 2, as 6-15 centroblasts/ hpf; and grade 3, as > 15 centroblasts/hpf.[6] Clinically, the behavior and outcome of grades 1 and 2 follicular lymphoma are similar, and both are considered indolent lymphomas. In contrast, grade 3 is a more aggressive disease.[7] Patients usually present with diffuse, painless, persistent, generalized lymphadenopathy, and except for bone marrow involvement, extranodal disease is uncommon. The size of the lymph nodes may vary with time, and complete disappearance followed by reappearance has been reported. The Ann Arbor staging system developed in 1971 for Hodgkin's disease has been adapted for staging NHL as well.[8,9] In a retrospective analysis, Anderson et al found that clinically, 66% of patients had stage III disease, whereas pathologically, 84% of patients had stage III/IV disease at presentation.[10]
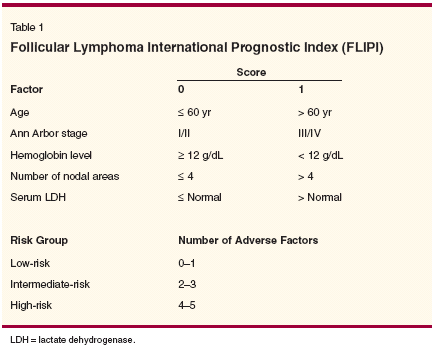
Prognostic Features A large number of features have been associated with an adverse outcome in follicular lymphoma, including increased age, male gender, stage of disease, performance status, presence of B symptoms, elevated levels of serum lactate dehydrogenase (LDH), serum beta-2 microglobulin, and hemoglobin, as well as bulk disease and extranodal involvement. The most widely used system is the International Prognostic Index (IPI). According to the IPI, age > 60 years, stage III/IV disease, Eastern Cooperative Oncology Group (ECOG) performance status < 2, involvement of more than two extranodal sites, and elevated serum LDH levels are considered adverse prognostic factors.[ 11] In a Spanish study, 10-year overall survival rates were 74% for low, 45% for low-intermediate, 54% for high-intermediate, and 0% for high-risk groups based on IPI score (P < .001).[12] In 1991, Romaguera et al developed a model based on the tumor burden to predict outcomes in follicular lymphoma. The investigators included number of extranodal sites involved, degree of bone marrow involvement, and lymph node size in this model, and found that patients with a low tumor burden had a 10-year survival of 73% as compared to 24% for those with a high tumor burden.[ 13] Decaudin et al studied 484 patients with stage III/IV follicular lymphoma, identifying three prognostic factors for poor overall survival: B symptoms, age greater than 60 years, and at least three nodal sites greater than 3 cm.[14] Frederico et al devised a prognostic model based on age, gender, number of extranodal sites, B symptoms, serum LDH level, and erythrocyte sedimentation rate. They found a 5- and 10-year survival rate of 90% and 65% for patients at low risk, respectively; 75% and 54% for patients at intermediate risk; and 38% and 11% for those at high risk.[15] In a recent retrospective analysis of 810 patients treated with anthracycline- based chemotherapy and adjuvant radiotherapy to sites of initial bulky nodal disease, only three factors- age > 60 years, presence of B symptoms, and involvement of more than two extranodal sites-were found to influence overall and progressionfree survival. When the IPI was applied to these patients, no statistical differences were observed in outcomes between the various groups. This suggests a lack of uniform prognostic factors for follicular lymphoma, indicating an urgent need for multicentric international clinical analysis to define prognostic factors.[16] Recently, an international group looked at a variety of prognostic factors in a large number of patients with follicular lymphoma and found that age ≥ 60 years, stage III/IV disease, elevated LDH level, hemoglobin < 12 g/dL, and number of nodal sites ≥ 5 had a significant adverse effect on survival. They designed an index based on these factors: the Follicular Lymphoma International Prognostic Index (FLIPI). Based on the FLIPI, patients could be divided into three prognostic groups: good (0-1 adverse prognostic factor), intermediate (2 factors), and poor (≥ 3 factors). The 10-year overall survival was 70.7%, 50.9%, and 35.5%, respectively for the three groups (Table 1).[17] The role of histopathology in predicting outcome is much more controversial. It is generally agreed that the presence of large cells confer a more aggressive nature to follicular lymphoma.[18,19] The degree of nodularity has also been studied as a prognostic factor. In an ECOG study, a pure nodular pattern (defined as nodularity involving 75% or more of the cross-sectional area) was found to be an important favorable prognostic indicator as compared with a nodulardiffuse pattern.[20] Similarly, Hu et al from Stanford University found that patients with focally follicular areas (ie, < 25% of the histologic section) had significantly worse outcomes compared with patients with a predominantly follicular architecture (ie, > 50% of the section).[21] Another recent study from the University of Nebraska found that cases of follicular lymphoma (grade 3) with a predominant diffuse component (> 50%) had a significantly worse overall and event-free survival (similar to diffuse large B-cell lymphoma) than those without.[22] Martin et al studied the prognostic value of proliferative index in 106 patients with follicular lymphoma. They determined the proliferative index quantitatively using an automated image analyzer and found that patients with a low proliferative index (< 40%) had a significantly longer overall survival than those with a high proliferative index (≥ 40%), but the proliferative index did not predict failure-free survival.[23] Clinical Course Horning and Rosenberg studied 83 asymptomatic patients with low-grade NHL (most of whom had follicular lymphoma), in whom the advanced disease was initially managed without therapy. The 5- and 10-year actuarial survival rates were 82% and 73%, respectively, and spontaneous regressions occurred in 19 untreated patients.[ 24] Portlock and Rosenberg retrospectively studied 44 asymptomatic patients with stage III/IV NHL who received no initial treatment. They found that the median time to treatment was 31 months, and the median survival was 121 months. The 4-year actuarial survival was 77.3%.[25] More recently, in a retrospective study conducted at Stanford University, Advani et al studied 43 patients with stage I/II follicular lymphoma who were not treated initially. They showed in this study that deferred therapy is an acceptable approach for patients with early-stage disease.[26] Some authors have stated that conventional chemotherapy is neither curative nor does it substantially modify the natural course of follicular lymphoma.[ 27] Patients with disseminated disease ultimately die from the disease, with a median survival time of 8 to 10 years.[28] However, longterm disease-free survival (relapsefree survival of ~50%) has been noted in a number of settings, ie, limitedstage disease, grade 3 follicular lymphoma (unpublished data), achievement of complete remission following chemotherapy or concomitant chemotherapy and radiation therapy (RT), and following autologous stem cell transplantation.[29-34] Whether one considers follicular lymphoma to be curable depends on the definition of cure. If one considers clinical cure (ie, the absence of a clinically obvious relapse) as the criterion, this has been demonstrated in various groups of patients who have had a long-term disease-free survival following various interventions as mentioned above. However, since follicular lymphomas have an extremely indolent course, it is very difficult to determine if clinical cure corresponds to complete elimination of all lymphoma cells. In addition, patients in clinical remission can have small numbers of circulating lymphocytes exhibiting t(14;18) translocations that are considered pathognomonic of follicular lymphoma.[35] However, these cells can also be identified in the peripheral blood of healthy individuals. Schuler et al found that if the sensitivity of the assay used for detection was high enough in almost all healthy individuals, one or multiple cell clones carrying the t(14;18) translocation could be found.[36] Thus, long-term disease-free survival is possible after treatment of patients with follicular lymphoma, but it is impossible to be sure that this corresponds to the absence of even a single lymphoma cell in the patient. Very late relapses suggest that some patients might harbor quiescent lymphoma cells for very long periods of time. Treatment of Limited-Stage DiseaseNo Initial Treatment
In a French study, Brice et al randomized 193 newly diagnosed follicular lymphoma patients with a low tumor burden to no initial treatment, prednimustine, or interferon alfa-2b (Intron A) and found no difference in the overall survival rate at 5 years among the three groups.[37] In the Stanford study described above, Advani et al found that at a median follow-up of 86 months, 27 patients (63%) had not yet required treatment.[26] Radiation Therapy
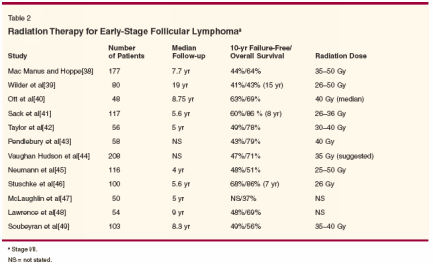
Radiation therapy has been the mainstay of treatment for limited-stage grade 1 and 2 follicular lymphoma. Table 2 presents an overview of the clinical trials that have used RT as the major modality for treatment.[38-49] The results of these studies uniformly show that involved-field RT confers a 10-year failure-free survival of approximately 45% in patients presenting with early-stage disease. Chemotherapy
The role of chemotherapy alone in the treatment of early-stage follicular lymphoma is not exactly clear. Jeffery et al treated 30 patients with nonbulky stage I, nodal, intermediate-grade NHL with RT. They then compared the outcomes in 11 patients with bulky stage I disease treated with combination chemotherapy. They found that the 5-year actuarial survival for the 30 patients treated with RT was 86%, as compared to a 60% 4-year actuarial survival in the 11 patients treated with chemotherapy.[50] In contrast, Teczan et al evaluated 40 patients with previously untreated follicular lymphoma and found that stage IA patients treated with chemotherapy (with or without RT) showed a better trend for 10-year event-free survival as compared to RT alone. There was, however, no difference in the estimated 10-year overall survival.[51] Combined Chemoradiation
In a study conducted at Memorial Sloan-Kettering Cancer Center, Yahalom et al randomized 44 patients with clinical or pathologic stage I intermediate-grade or low-grade NHL to receive regional RT alone or regional RT followed by six cycles of CHOP chemotherapy (cyclophosphamide [Cytoxan, Neosar]/doxorubicin HCl/vincristine [Oncovin]/prednisone). They found an 83% actuarial relapse-free survival rate for the RT-plus-CHOP group at 7 years, compared with 47% for the RT-alone group. The overall survival for the two groups was 88% and 66%, respectively. However, in patients with low-grade NHL, the addition of adjuvant CHOP did not improve outcomes.[52]
McLaughlin et al prospectively treated 44 patients with stage I/II lowgrade lymphoma with sequential chemotherapy and involved-field RT. At a median follow-up of 32 months, they had 5-year overall and failurefree survival rates of 89% and 74%, respectively.[53] Seymour et al studied 102 eligible patients with stage I/II low-grade lymphoma (85 patients with follicular lymphoma) treated with chemotherapy and involved-field RT. They found that the 10-year time to treatment failure and overall survival rates in patients with follicular lymphoma were 72% and 80%, respectively.[ 54] These results constitute a marked improvement in the 10-year disease-free survival of 41% to 64% reported by the various studies involving RT alone described above. Richards et al retrospectively studied 202 patients with clinical stage I/II NHL between 1972 and 1985. They found that although the duration of remission was better in patients who received adjuvant chemotherapy than in those treated with RT alone, there was no difference in overall survival between the two groups of patients. Since neither of these were randomized trials, it is unclear whether the addition of chemotherapy would improve the outcomes obtained by RT alone.[55] Treatment of Advanced DiseaseNo Initial Treatment
The exact time to initiate treatment in this setting is controversial, since these patients have a prolonged survival despite frequent relapses. In an effort to answer the question of whether early aggressive therapy is superior to watchful waiting, the National Cancer Institute (NCI) conducted a study in 104 patients with advanced indolent lymphomas. They randomly assigned 44 patients to the watchful waiting group, in which only carefully defined, limited RT was administered if necessary; 45 were randomly assigned to aggressive combinedmodality treatment with ProMACEMOPP (prednisone, methotrexate, doxorubicin, cyclophosphamide, etoposide, mechlorethamine [Mustargen], vincristine, procarbazine [Matulane], prednisone), followed by total nodal irradiation. They found no difference in overall survival at 5 years (> 75% in each group), but diseasefree survival was better in the group that received initial therapy.[56] In a recently published British study, Ardeshna et al randomized 309 patients with asymptomatic, advancedstage, low-grade NHL (204 patients with follicular lymphoma) to immediate systemic chemotherapy with chlorambucil (Leukeran), 10 mg/d continuously, vs an initial policy of observation with systemic therapy delayed until disease progression. However, in contrast to the above study, they found that overall survival and cause-specific survival did not differ between the two groups.[57] Chemotherapy
- CVP-Systemic chemotherapy is the mainstay of treatment in patients with advanced-stage follicular lymphoma. Hoppe et al randomized 51 patients with favorable-histology NHL (pathologic stage III/IV disease) to single-alkylating agent chemotherapy, combination chemotherapy with CVP (cyclophosphamide, vincristine, prednisone), or fractionated wholebody irradiation followed by low-dose involved-field irradiation. They found an actuarial survival of 84% at 4 years, with similar survival observed for each of the three treatment options.[58]
- Kennedy et al randomized 58 patients with advanced lymphoma to the CVP combination or these same three agents given separately in succession. They demonstrated a complete remission rate of 81% with the combination and 46% with sequential use of the three agents.[59] Portlock et al randomized 63 previously untreated patients with stage IV NHL with favorable histologies to three groups: CVP alone, split-course CVP and total lymphoid irradiation, or single- alkylating agent therapy. The actuarial probability of obtaining a complete remission was greater than 80% in all three groups. In contrast to the above findings of Kennedy et al, they found no statistically significant differences among the groups in terms of the probability of disease-free or overall survival.[60]
- CHOP-Peterson et al randomized 228 patients with stage III/IV follicular lymphoma to cyclophosphamide or the CHOP-B combination (cyclophosphamide, doxorubicin, vincristine, prednisone, bleomycin [Blenoxane]). These investigators observed complete responses in 66% of those treated with cyclophosphamide and in 60% of those treated with CHOP-B. They found no difference in overall survival between the two groups. However, in an unplanned subgroup analysis, patients with follicular mixed lymphoma who received the combination experienced improved disease control and survival.[61]
- Jones et al compared two CHOP regimens (CHOP with either low-dose bleomycin or bacille Calmette-Gurin [BCG] by scarification) to COP-Bleo (cyclophosphamide/vincristine/prednisone, with low-dose bleomycin). In patients with follicular lymphoma, they found no difference in complete response rates, relapse-free survival, and overall survival among the three groups.[62]
- Other Combinations-In an effort to improve outcomes in this setting, other regimens have been tried. Ezdinli and associates analyzed 252 patients with advanced-stage favorable NHL treated with moderate-CP (cyclophosphamide, prednisone)-vs intensive- BCVP (carmustine [BCNU, Gliadel], cyclophosphamide, vincristine, prednisone) or COPP (cyclophosphamide, vincristine, procarbazine, prednisone)-chemotherapy regimens. They found an overall complete response rate of 57% and a median duration of remission of 88 weeks. There was no difference in the response rate, response duration, or survival rate among the various groups.[63]
- In a recent Italian study, Zinzani et al performed a comparative trial of FM (fludarabine [Fludara], mitoxantrone [Novantrone]) with CHOP as front-line chemotherapy, with and without sequential rituximab (Rituxan). They randomized 140 previously untreated patients with grades 1 and 2 follicular lymphoma to either CHOP or FM. The overall clinical response was the same in both groups (FM: 96%; CHOP: 98%). However, the complete response rate was higher in the FM arm (68% vs 42%; P = .003). They also found that the percentage of patients with a negative bcl-2/immunoglobulin heavy chain (IgH) status by qualitative polymerase chain reaction was higher in the FM group (47% vs 29%; P = .03). These results seem to indicate that FM may be superior to CHOP for front-line therapy of follicular lymphoma. However, it is unclear whether this superiority translate into superior progression-free or overall survival.[64]
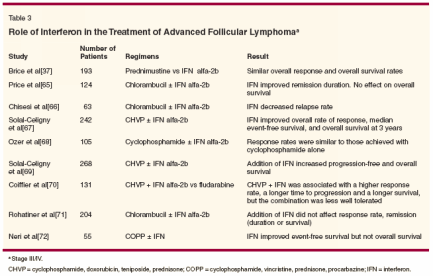
- Interferon-Interferon has been tried alone and in combination with cytotoxic chemotherapy for the treatment of advanced follicular lymphoma. The individual studies are summarized in Table 3.[37,65-72] Rohatiner et al performed a meta-analysis of these clinical trials involving interferon. Their initial meta-analysis showed an overall survival difference in favor of interferon, but a significant heterogeneity effect suggested significant differences between trials.
- The investigators then divided the trials based on the interferon dose used and found that trials using a more intensive therapy showed a large and significant survival advantage in favor of interferon, with a 14% survival difference at 5 years (74% vs 60%) and a 19% survival difference at 8 years (57% vs 38%). However, trials that used a low-intensity interferon regimen showed no survival difference.[ 73] The results with the use of interferon are mixed, thereby leading to controversy about the exact role of interferon in the treatment of advanced follicular lymphoma.
- Fludarabine-The purine analog fludarabine has been shown to be active against follicular lymphoma. In a phase I study, McLaughlin et al administered a combination of fludarabine, mitoxantrone, and dexamethasone (FMD) to 21 patients with recurrent low-grade lymphoma or follicular largecell lymphoma. They obtained a response rate of 71%, with a 43% complete remission rate.[74] The Groupe d'Etude des Lymphomes de l'Adulte (GELA) workers used fludarabine as first-line therapy in 55 patients with advanced disease, demonstrating an overall response rate of 65% and a complete response rate of 37%.[75]
- Emmanouilides et al treated 25 patients with advanced indolent lymphoma using a combination of fludarabine and mitoxantrone, without corticosteroids. They demonstrated a response rate of 84%; at a median follow-up of 22 months, the median survival was not yet reached.[76] Coiffier et al compared fludarabine alone with a combination of CHVP (cyclophosphamide, doxorubicin HCl, teniposide [Vumon], prednisone) plus interferon in elderly patients. They found that although fludarabine was better tolerated, CHVP plus interferon was associated with a higher response rate, a longer time to progression, and a longer survival.[70] In an Italian study, Zinzani et al compared fludarabine alone with a combination of fludarabine and idarubicin (Idamycin). They found that although the initial complete response rates were similar in both groups, the combination was more likely to confer a longer-lasting complete response.[ 77] Crawley and associates studied FMD in 54 patients with follicular lymphoma, most of whom had relapsed following prior treatment. The overall response rate was 69%, with complete responses seen in 20%. There was no difference between newly diagnosed and previously treated patients. However, the response rate was higher in patients with a chemosensitive relapse (84%) compared to patients in whom the last prior regimen had failed (44%).[78] Rohatgi et al conducted a phase II trial of sequential therapy with fludarabine followed by cyclophosphamide, mitoxantrone, vincristine, and prednisone in 27 patients with bulky stage II, III, or IV follicular lymphomas. They achieved a complete response in 67% and a partial response in 30%. Median relapse-free survival was 34 months, and at a median follow- up of 50 months, median survival was not reached for the entire cohort.[79] In a randomized phase III trial, Klasa et al compared the safety and efficacy of fludarabine and CVP in previously treated patients with recurrent progressive low-grade NHL. They found that at 2 years, progression- free survival (32% vs 14%; P = .028) and treatment-free survival (41% vs. 20%; P = .034) were higher in the fludarabine group. However, there was no difference in overall survival between the two groups (P = .738), and there were an increased number of treatment-related deaths in the fludarabine group.[80]
Unlabeled Antibodies
- Single-Agent Rituximab-Monoclonal antibody therapy has received much attention for the treatment of NHL, with a major focus on the indolent lymphomas. Rituximab, a chimeric antibody that targets CD20+ B cells has been extensively studied in the treatment of follicular lymphoma. Hainsworth et al treated 62 patients diagnosed with indolent lymphoma (38 patients with follicular lymphoma) and no previous chemotherapy, with weekly rituximab for 4 weeks every 6 months. During an interim analysis at week 6, 28 of 60 evaluable patients (47%) had an objective response, with 7% of patients achieving a complete response.[81] With continued maintenance, the final response rate increased to 73%, with 37% complete responses at a minimum follow-up of 24 months.[82] In a phase II trial, Colombat and associates used weekly rituximab as a firstline agent in 54 patients with follicular lymphoma and a low tumor burden. They achieved a response rate of 73% (36/50) at day 50. Of these 36 patients, 10 (27%) had progressive disease at the end of 1 year.[83]
- In a British study, Foran et al treated 70 patients with previously treated follicular lymphoma using weekly rituximab for four doses. They obtained an overall response rate of 46%, with only 2 of 70 patients achieving a complete response. The median duration of response was 11 months.[84]
- Postchemotherapy Rituximab- Due to the excellent response obtained with single-agent rituximab, various studies have been performed using rituximab as consolidation therapy following cytotoxic chemotherapeutic agents. In the study conducted by Hainsworth, repeated courses of rituximab increased the response rate from 47% after the initial course to 65%, and the complete response rate from 7% to 27%.[81] In an Austrian study using initial CHOP chemotherapy followed by rituximab, Jaeger et al found that 10 of 24 patients (41.7%) achieved a complete response after chemotherapy. An additional 10 patients achieved a complete response after additional rituximab therapy. Rituximab thus led to complete response in 10 of 14 patients (71%) who did not do so with CHOP alone.[85]
- The Southwest Oncology Group (SWOG) conducted a similar study using a combination of six cycles of CHOP followed by four cycles of rituximab in patients with newly diagnosed follicular lymphoma. They evaluated 84 patients for response and found that 54% had a complete response and 18% a partial response for an overall response rate of 72%.[86] In the Italian study by Zinzani et al mentioned above, the investigators treated those patients who obtained a complete response but remained bcl-2/IgH-positive and those who achieved a partial response (irrespective of their bcl-2/IgH status) after initial chemotherapy with four doses of rituximab. They found that the immunotherapy phase led to a significant improvement in the complete response rate (57% after chemotherapy to 76% after chemotherapy plus rituximab; P = .000) and combined clinical and molecular response (29% to 61%; P = .000).[64]
- Combined Immunochemotherapy- Given the success of combined immunochemotherapy in diffuse large B-cell lymphoma, investigators have assessed the combination of rituximab with cytotoxic chemotherapeutic agents in follicular lymphoma. Czuczman et al conducted a study to determine the safety and efficacy of the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) in this setting. They treated 38 patients with follicular lymphoma using six cycles of CHOP and rituximab. Twenty-two patients (57.9%) had a complete response, and 16 patients (42.1%) had a partial response. One hundred and six months after the first treatment, remission was ongoing in 19 of the 38 patients (remission duration: 77.3+ to 105.6+ months). The median time to progression in this study was 82.3 months.[87]
- Two recent European trials compared chemotherapy alone with chemotherapy in combination with immunotherapy. The German Low Grade Lymphoma Study Group (GLSG) compared the efficacy of standard CHOP chemotherapy with that of R-CHOP in 606 patients with follicular lymphoma. They found that although the addition of rituximab led only to a moderate improvement in the complete response rate (21% vs 18%) and overall response rate (97% vs 93%) in comparison to chemotherapy alone, median time to treatment failure was significantly increased after R-CHOP as compared to CHOP (not reached vs 2.7 years; P < .0007).[88] In a similar phase III trial, Marcus et al compared the addition of rituximab to CVP chemotherapy to CVP alone in the therapy of 322 previously untreated patients with stage III/IV CD20+ follicular lymphoma. Their results were similar to those found in the GLSG study. The overall response rate and complete response rate were 81% and 40% in the R-CVP arm vs 57% and 10% in the CVP arm, respectively (P < .0001). With a median follow-up of 18 months, R-CVP recipients had a highly significan prolonged time to treatment failure (median: 27 vs 7 months; P < .0001). The median time to progression also was prolonged in patients receiving R-CVP (not reached vs 13 months; P < .0001).[89]
Radiolabeled Antibodies
Several anti-CD20 radioimmunoconjugates are being evaluated for the treatment of indolent NHL. Radioimmunotherapy combines immune and radiotherapeutic mechanisms to target and destroy tumor cells. The advantage of these agents is that they deliver targeted therapy to the tumorbearing areas. Radioimmunotherapy with yttrium (Y)-90 and iodine (I)-131- labeled anti-CD20 antibodies (ibritumomab tiuxetan [Zevalin] and tositumomab/ I-131 tositumomab [Bexxar], respectively) has a high rate of tumor response in patients with relapsed or refractory, low-grade, follicular, or transformed B-cell NHL.
- Ibritumomab-Witzig et al compared Y-90 ibritumomab tiuxetan with a control immunotherapy (rituximab) in 143 patients with relapsed or refractory low-grade, follicular, or transformed CD20+ transformed NHL in a randomized phase III trial. Patients receiving Y-90 ibritumomab tiuxetan had significantly higher overall (80% vs 56%) and complete (30% vs 16%) response rates, as compared to those receiving rituximab.[90] In a phase II study, Wiseman and associates evaluated the safety and efficacy of a reduced dose of Y-90 ibritumomab tiuxetan in 30 patients with mild thrombocytopenia who had advanced, relapsed, or refractory, low-grade, follicular, or transformed B-cell NHL. This group had an overall response rate of 83% with a 43% complete response rate, thereby demonstrating that even reduced-dose ibritumomab tiuxetan is safe, efficacious, and well tolerated.[91]
- Ibritumomab tiuxetan has been shown to be efficacious even in patients refractory to rituximab. In a multicenter study, Witzig and colleagues used ibritumomab tiuxetan in 54 patients with follicular lymphoma who had no objective response to rituximab. The overall response rate was 74%, with 15% complete response.[ 92] In a phase I trial, Vose et al treated 16 patients with Y-90 ibritumomab who had relapsed after highdose chemotherapy and autologous stem cell transplantation. They found an overall response rate of 45% (2 complete responses, 3 partial responses), demonstrating that this agent can be effective even in the posttransplant setting.[93]
- Tositumomab-A multicenter phase II study evaluated the efficacy and safety of I-131 tositumomab in patients with relapsed or refractory low-grade or transformed low-grade CD20+ NHL. Of the 45 patients treated with I-131 tositumomab, 27 (57%) had a response and 15 (32%) had a complete response.[94] In a phase I/II single-center study conducted at the University of Michigan, Kaminski and colleagues treated 59 relapsed or refractory patients with I-131 tositumomab. They had a response rate of 71% with a complete response rate of 34%. During long-term follow-up, seven patients remained in complete response for 3 to 5.7 years. Of the 16 patients who were re-treated on progression, 9 responded and 5 had a complete response.[95]
- In a multicenter study, Kaminski et al compared the efficacy of I-131 tositumomab to the patients' last qualifying chemotherapy regimens. They treated 60 patients who had not responded or progressed within 6 months after their last qualifying chemotherapy with a single course of I-131 tositumomab. They observed a higher response rate (65% vs 28%) and complete response rate (20% vs 3%) after I-131 tositumomab, compared with their last qualifying chemotherapy. Also, the median duration of response was higher after I-131 tositumomab (6.5 vs 3.4 months).[96] This response to I-131 tositumomab seems to be similar in both rituximab-refractory/relapsed and rituximab- naive patients. Coleman et al evaluated 230 patients treated with tositumomab/I-131 tositumomab and found that 11 out of 40 patients (28%) in the rituximab-refractory/relapsed group and 44 out of 190 (23%) in the rituximab-naive group had a durable complete response.[97] In a British phase II trial, Davies et al studied the efficacy and safety of tositumomab/I-131 tositumomab at recurrence of indolent lymphoma or transformed indolent B-cell lymphoma. Patients had an overall response of 76% (31/41) with 20 (49%) achieving a complete response.[98] Recently, the role of I-131 tositumomab following chemotherapy for previously untreated follicular lymphoma has been investigated. A SWOG protocol treated 90 patients with six cycles of CHOP followed 4 to 8 weeks later by tositumomab/ I-131 tositumomab. The overall response rate to the entire treatment regimen was 90%, including a complete response rate of 67%. Of the patients who achieved less than a complete response with CHOP, 57% improved their status after radioimmunotherapy. At a median follow-up of 2.3 years, the estimated 2-year progression-free and overall survival were excellent (81% and 97%, respectively).[99] Kaminski et al conducted a phase II trial to evaluate the efficacy of I-131 tositumomab therapy in 76 patients with previously untreated advanced- stage follicular lymphoma. They obtained an overall response in 74 of 76 patients (97%), with 48 (63%) achieving a complete response and 26 (34%), a partial response.[100]
Bone Marrow or Stem Cell Transplantation
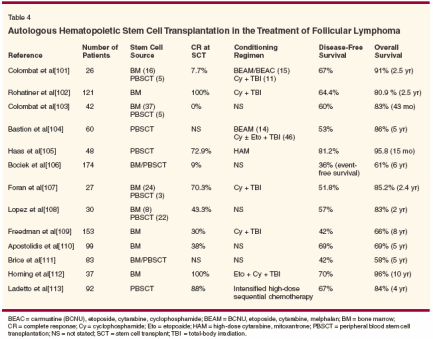
In contrast to aggressive NHL, the exact role of hematopoietic stem cell transplantation (HSCT) in relapsed follicular lymphoma is unclear. Table 4 summarizes the various clinical trials that have evaluated the role of HSCT in this disease.[101-113]
- Autologous Transplantation- Patients undergoing autologous HSCT have had a significantly longer diseasefree survival than patients undergoing conventional chemotherapy alone. In the GELA study,[111] Brice et al demonstrated that patients who underwent HSCT had a significantly increased 5-year disease-free (42% vs 16%) and overall survival (58% vs 38%) as compared to those who received standard treatment alone.
- In a novel approach, Recher et al designed a double intensive regimen supported by autologous stem cell transplantation in 36 patients with follicular lymphoma. They gave their patients two monthly cycles of CHOP and granculocyte-macrophage colony- stimulating factor (GM-CSF, Leukine), each followed by peripheral blood stem cell collection and then first intensification with melphalan (Alkeran) supported by HSCT and GM-CSF. This was followed by a bone marrow harvest and second intensification with cyclophosphamide and total-body irradiation supported by the bone marrow harvest, HSCT, and GM-CSF. They then maintained these patients on interferon-alfa 3 days a week. They found a complete response rate of 94% (34/36) at the end of the procedure. After a median follow- up of 86 months, the estimated 10-year disease-free and overall survival rates were 60% and 83%, respectively.[ 114]
- The introduction of radioimmunoconjugates to the therapeutic armamentarium for follicular lymphoma has led to increased interest in the use of these agents as part of the conditioning regimen prior to HSCT. Gopal et al performed a multivariable comparison of 125 patients diagnosed with follicular lymphoma and treated with either high-dose radioimmunotherapy using I-131 tositumomab (n = 27) or conventional high-dose therapy (n = 98) and autologous HSCT. Patients who underwent high-dose radioimmunotherapy had an improved progression- free and overall survival. The estimated 5-year overall and progression- free survival rates were higher in the radioimmunotherapy group as compared to the conventional high-dose chemotherapy group (67% and 48%, vs 53% and 29%, respectively).[115] Although our experience at the University of Nebraska shows a 45% 10-year event-free survival rate (unpublished data), most authors believe that there is no plateau in disease-free survival following autologous HSCT. This has led investigators to evaluate the role of purging the bone marrow/ stem cells prior to transplantation. In a large registry study, van Besian et al compared outcomes following purged (n = 131) and unpurged (n = 597) autologous HSCT for follicular lymphoma. They found no difference in 5-year recurrence rates and overall survival between the two groups. However, patients who underwent a purged autologous HSCT had a 26% lower recurrence risk than those who underwent an unpurged autologous HSCT.[116] Schouten et al conducted an international, randomized clinical trial in 140 patients with follicular lymphoma to determine whether high-dose therapy followed by autologous HSCT was more effective than standard treatment. They also assessed the effect of purging of the stem cell graft on progression- free and overall survival. They found that patients receiving chemotherapy alone had a significantly inferior progression-free survival, compared to patients who received autologous HSCT, both purged and unpurged (hazard ratio = 0.44 and 0.36, respectively). There was also a significant reduction in the hazard rates for overall survival when the two transplantation arms were combined and compared with the chemotherapy-alone group. They found that patients who received a purged transplant had a similar progression-free survival to those who received an unpurged transplant (hazard ratio = 1.01; 95% confidence interval = 0.5-2.04).[117]
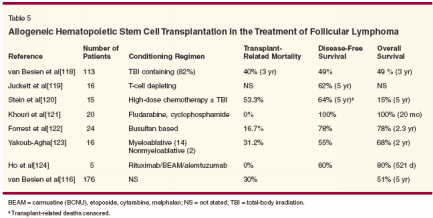
- Allogeneic Transplantation- The increased incidence of late relapse following autologous HSCT has led to an increased interest in exploring the possibility of an allogeneic HSCT for the treatment of follicular lymphoma (Table 5).[116,118-124] Although the risk of relapse following allogeneic HSCT is low, the increased incidence of transplant-related mortality and acute and chronic graftvs- host disease (GVHD) contribute to the absence of a significant difference in overall outcomes when compared to autologous HSCT.
- The Verdonck et al study of 28 patients with advanced low-grade lymphoma compared the results of allogeneic and autologous HSCT in 28 patients with advanced low-grade lymphoma. Allogeneic transplant recipients had a much lower probability of disease progression (0% vs 83%) and a significantly higher progressionfree survival at 2 years (68% and 22%).[125] van Besian et al compared 5-year recurrence rates and overall survival between recipients of autologous and allogeneic HSCT for follicular lymphoma. In their study, 5-year recurrence rates in patients undergoing allogeneic HSCT were significantly lower than those undergoing either purged or unpurged autologous HSCT (21% vs 43% and 58%). However, there was no difference in the 5-year probabilities of survival (51%, 62%, and 55%) among the three groups.[116] In a similar study at the University of Nebraska, Deshpande et al compared long-term outcomes of 204 patients who received autologous or allogeneic HSCT for follicular lymphoma. They found that allogeneic HSCT led to a significant benefit in 5-year progression-free survival (76% vs 41%), but not in overall survival (76% vs 61%).[126] Treatment of relapses following allogeneic HSCT has involved donor lymphocyte infusion. In a pilot study, van Besian et al treated nine adults who relapsed following an allogeneic HSCT for NHL using withdrawal of immunosuppression and donor lymphocyte infusion. Four out of nine patients responded to withdrawal of immunosuppression. A minor response was observed in one of three recipients of donor lymphocyte infusions.[ 127] In a Dutch study, Mandingers et al treated seven patients with indolent lymphoma who had relapsed following an allogeneic HSCT using donor lymphocyte infusion. They obtained an immediate response in six of seven patients. In the seventh patient, chemotherapy followed by another donor lymphocyte infusion resulted in a complete response.[128] The lower relapse rates and the presence of an apparent plateau in the survival curves after the initial posttransplant period in allogeneic HSCT indicate a possible curative potential for this treatment modality in patients with follicular lymphoma. However, the optimal time of transplant and the conditioning regimen still need to be evaluated in clinical trials.
Future DirectionsVaccines
The B-cell lymphomas express a tumor-specific clonal immunoglobulin (idiotype). This is composed of the unique antigenic determinants in the variable regions of the clonal immunoglobulin expressed by the tumor cells. This can be recognized by the immune system and can serve as a target for active immunotherapy. Hsu et al treated 41 patients with B-cell NHL using a vaccine consisting of tumor immunoglobulin protein coupled to keyhole limpet hemocyanin. In their series, 20 patients generated specific immune responses against the idiotypes of their tumor immunoglobulin. Patients who mounted an immune response had increased progression- free survival (7.9 vs 1.3 years) and overall survival (median survival not reached vs 7 years), as compared to those who did not mount an immune response.[129] Timmerman et al vaccinated 35 patients with dendritic cells pulsed with tumor-derived idiotype protein. Of the 23 patients who completed the vaccination schedule, 15 mounted an immune response, either T-cell- mediated or immune-mediated. At a median of 43 months after chemotherapy, 16 of 23 patients showed no tumor progression. Six patients who experienced progression received booster doses of the vaccine, and three of them had a response.[130] Newer Monoclonal Antibodies
The success of the monoclonal antibody rituximab and the radioimmunoconjugates tositumomab and ibritumomab has spurred research into the development of newer targeted therapies directed toward NHL. Epratuzumab (anti-CD22) and apolizumab (Hu1D10) seem most promising in this regard. Epratuzumab is a humanized monoclonal antibody directed against the CD22 determinant RFB4, which is present on 75% of B lymphocytes.[131] In a phase I/II trial, Leonard et al treated 55 patients with escalating doses of epratuzumab and obtained objective responses in 24% of patients with follicular lymphoma. A 43% objective response rate was seen in follicular lymphoma patients treated at 360 mg/m2/wk, suggesting that this dose should be explored further.[132] Postema et al tried to determine the maximum tolerated dose of a new radioimmunoconjugate created by a combination of epratuzumab with rhenium (Re)-186 in a phase I study. They found that Re-186 epratuzumab at a dose of 2.0 GBq/m2 was well tolerated. Five out of 15 patients with NHL of diverse histology showed objective responses following a single dose of Re-186 epratuzumab.[133] Apolizumab is a humanized IgG1 monoclonal antibody that binds to a variant (likely posttranslational modification) of the HLA-DRB chain.[134] Apolizumab induces antibody- dependent cellular cytoxicity and complement-mediated lysis as well as signaling via tyrosine phosphorylation in lymphoma cell lines, and hence, it is being evaluated in NHL.[135] In a phase I study conducted at the NCI, 20 patients were treated at various dose levels. The toxicity observed was mainly grade 1 and 2, and occasionally grade 3. The investigators found clear evidence of antitumor effects, especially in follicular lymphoma, where four of eight patients demonstrated an objective response.[136] Galiximab is a macaque-human chimeric antibody directed against CD80. CD80 is an immune costimulatory molecule expressed on the surface of a wide variety of hematologic malignancies, including follicular lymphoma.[ 137] Czuczman et al conducted a phase I/II trial evaluating the efficacy of galiximab in the treatment of relapsed/ refractory follicular lymphoma. They treated 37 patients with escalating doses of galiximab and found that the agent was well tolerated with no major adverse effects. They observed two complete responses and one partial response in 34 evaluable patients, for an overall response rate of 9%.[137] In another phase I study, Gordon et al combined escalating doses of galiximab with rituximab in patients with relapsed/refractory follicular lymphoma. They enrolled 12 patients into this phase of their study, and at 50 days, they observed three complete and four partial responses. They also found no major adverse effects or dose-limiting toxicities.[138] These results show that galiximab is a potentially safe and effective treatment for relapsed/refractory follicular lymphoma. Antisense Therapy
The bcl-2 gene is commonly overexpressed in NHL.[139] More than 85% of patients with follicular lymphoma and t(14;18) overexpress bcl-2.[140] This leads to resistance to apoptosis, thereby promoting tumorigenesis.[ 139] Waters et al conducted a phase I study to evaluate the efficacy and toxicity of an antisense oligonucleotide targeting bcl-2 in patients with NHL. Among the 21 patients they treated with the oligonucleotide, there was one complete response, two minor responses, nine cases of stable disease, and nine cases of progressive disease, thereby demonstrating that antisense therapy was feasible and potentially effective in NHL.[141] Proteasome Inhibitors
The proteasome is a multienzyme complex that is present in all eukaryotic cells. It degrades proteins that regulate cell-cycle progression and causes proteolysis of the endogenous inhibitor of nuclear factor (NF)- kappaB.[142] Bortezomib (Velcade) is a boronic acid derivative that is a highly selective, potent, reversible proteasome inhibitor. The initial demonstration of in vivo antitumor activity of a proteasome inhibitor used a human lymphoma xenograft model,[ 143] leading to an interest in the use of this agent in lymphoid malignancies. O'Connor et al administered bortezomib to 21 previously treated patients with relapsed or refractory indolent lymphomas (small lymphocytic lymphoma/chronic lymphocytic leukemia type [n = 3], follicular lymphoma [n = 9], mantle cell lymphoma [n = 8], and nodal marginal zone lymphoma [n = 1]). They found that six of the eight evaluable patients with follicular lymphoma achieved a major response, with one patient obtaining a durable complete response.[144] CpG Oligonucleotides
Bacterial DNA and synthetic oligodeoxynucleotides containing unmethylated cytosine-guanine dinucleotides known as cytosine phosphorothioate guanine (CpG) oligodeoxynucleotides can activate immune-cell subsets, including cells that participate in antibody- dependent cell-mediated cytotoxicity. Through these effects, CpG oligonucleotides could potentially augment the antitumor effects of monoclonal antibodies. Indeed animal studies have shown that CpG oligonucleotides enhance the efficacy of antitumor monoclonal antibody therapy in the 38C13 murine B-cell lymphoma.[145] Friedberg et al conducted a phase I study combining escalating doses of an immunostimulatory CpG oligonucleotide (1018 ISS) with rituximab in 16 patients with relapsed/refractory NHL (13 patients with follicular lymphoma). The regimen was well tolerated with no major toxicities, producing one complete response, five partial responses, and two cases of progressive disease. Interestingly, these investigators also found an increase in the expression of several interferon-inducible genes. This provides a rationale for further investigation of combination immunotherapy approaches in NHL.[146] Nonmyeloablative Stem Cell Transplantation
The biggest drawback to the routine use of allogeneic stem cell transplantation in the management of follicular lymphoma is the increased incidence of transplant-related mortality. The success of donor lymphocyte infusion in treating relapses following allogeneic HSCT has led to the thinking that a graft-vs-lymphoma (GVL) effect, mediated by lymphocytes, is at least partly responsible for the efficacy of allogeneic stem cell transplantation. Also supporting the existence of the GVL effect is the fact that patients who develop chronic GVHD after allogeneic HSCT have a lower probability of relapsing than patients who do not develop chronic GVHD.[147] In a study at the M. D. Anderson Cancer Center, Khouri et al treated 20 patients with indolent NHL with the combination of fludarabine, cyclophosphamide, and rituximab. They achieved a 2-year overall and relapsefree survival rate of 84%.[148] This study demonstrates the feasibility and efficacy of a relatively nontoxic, nonmyeloablative conditioning regimen for the treatment of follicular lymphoma. Conclusions In conclusion, long-term diseasefree survival is possible in select groups of patients with follicular lymphoma. Patients who have early-stage disease and those who achieve a complete response following initial chemotherapy tend to have long-term disease-free survival. The introduction of newer therapeutic modalities such as immunotherapy, radioimmunotherapy, and advances in stem cell transplantation has improved outcomes in follicular lymphoma over those seen with conventional chemotherapy and RT alone. However, because follicular lymphoma is an indolent disease, watchful waiting is still a good option in elderly asymptomatic patients with a low tumor burden. For patients who relapse following a complete or partial response, salvage chemotherapy followed by auto-HSCT improves outcomes as compared with conventional therapy. For younger patients with chemoresistant disease, allogeneic HSCT must be considered if patients have an HLAidentical sibling, as this strategy probably offers the best chance for cure in this subgroup. There is reason for excitement about the prospects for effective vaccine therapies for lymphoma, as randomized vaccine trials commence and newer cell-based vaccine trials enter the clinic. With such promising new prospects on the horizon, follicular lymphoma-long thought to be incurable- may soon have curative options, at least in a subset of patients.
Disclosures:
Dr. Armitage is a speaker for Genentech, GlaxoSmithKline, and Corixa. The other authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Armitage JO, Mauch PM, Harris NL, et al: Non-Hodgkin’s lymphomas, in DeVita VT, Hellman S, Rosenberg SA (eds): Cancer: Principles and Practice of Oncology, 6th ed, pp 2256-2316. Philadelphia, Lippincott Williams & Wilkins, 2001.
2. A clinical evaluation of the International Lymphoma Study Group classification of non- Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 89:3909-3918, 1997.
3. Armitage JO, Weisenberger DD: New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 16:2780-2795, 1998.
4. National Cancer Institute-sponsored study of classification of non-Hodgkin’s lymphomas: Summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathological Classification Project. Cancer 49:2112-2135, 1982.
5. Nathwani BN, Harris NL, Weisenberger DD, et al: Follicular lymphoma, in Jaffe ES, Haris NL, Stein H, et al (eds): World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, pp 162- 168. Lyon, IARC Press, 2001.
6. Mann RB, Berard CW: Criteria for the cytologic classification of follicular lymphomas: A proposed alternative method. Hematol Oncol 1:187-192, 1983.
7. Harris NL, Jaffe ES, Diebold J, et al: World Health Organization classification of neoplastic diseases haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 17:3835-3849, 1999.
8. Rosenberg SA: Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep 61:1023-1027, 1977.
9. Moormeier JA, Williams SF, Golomb HM: The staging of non-Hodgkin’s lymphomas. Semin Oncol 17:43-50, 1990.
10. Anderson T, Chabner BA, Young RC, et al: Malignant lymphoma. 1. The histology and staging of 473 patients at the National Cancer Institute. Cancer 50:2699-2707, 1982.
11. A predictive model for aggressive non- Hodgkin’s lymphoma. The International Non- Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329:987-994, 1993.
12. Lopez-Guillermo A, Montserrat E, Bosch F, et al: Applicability of the International Index for aggressive lymphomas to patients with low-grade lymphoma. J Clin Oncol 12:1343-1348, 1994.
13. Romaguera JE, McLaughlin P, North L, et al: Multivariate analysis of prognostic factors in stage IV follicular low-grade lymphoma: A risk model. J Clin Oncol 9:762-769, 1991.
14. Decaudin D, Lepage E, Brousse N, et al: Low-grade stage III-IV follicular lymphoma: Multivariate analysis of prognostic factors in 484 patients-a study of the groupe d’Etude des lymphomes de l’Adulte. J Clin Oncol 17:2499- 2505, 1999.
15. Federico M, Vitolo U, Zinzani PL, et al: Prognosis of follicular lymphoma: A predictive model based on a retrospective analysis of 987 cases. Intergruppo Italiano Linfomi. Blood 95:783-789, 2000.
16. Aviles A, Neri N, Cuadra I, et al: Lack of prognostic factors in follicular lymphoma. Leuk Lymphoma 44:143-147, 2003.
17. Solal-Celigny P, Roy P, Colombat P, et al: Follicular lymphoma international prognostic index. Blood 104:1258-1265, 2004.
18. Kantarjian HM, McLaughlin P, Fuller LM, et al: Follicular large cell lymphoma: Analysis and prognostic factors in 62 patients. J Clin Oncol 2:811-809, 1984.
19. Rodriguez J, McLaughlin P, Hagemeister FB, et al: Follicular large cell lymphoma: An aggressive lymphoma that often presents with favorable prognostic features. Blood 93:2202- 2207, 1999.
20. Ezdinli EZ, Costello WG, Kucuk O, et al: Effect of the degree of nodularity on the survival of patients with nodular lymphomas. J Clin Oncol 5:413-418, 1987.
21. Hu E, Weiss LM, Hoppe RT, et al: Follicular and diffuse mixed small-cleaved and large-cell lymphoma-a clinicopathologic study. J Clin Oncol 3:1183-1187, 1985.
22. Hans CP, Weisenburger DD, Vose JM, et al: A significant diffuse component predicts for inferior survival in grade 3 follicular lymphoma, but cytologic subtypes do not predict survival. Blood 101:2363-2367, 2003.
23. Martin AR, Weisenburger DD, Chan WC, et al: Prognostic value of cellular proliferation and histologic grade in follicular lymphoma. Blood 85:3671-3678, 1995.
24. Horning SJ, Rosenberg SA: The natural history of initially untreated low-grade non- Hodgkin’s lymphomas. N Engl J Med 311:1471-1475, 1984.
25. Portlock CS, Rosenberg SA: No initial therapy for stage III and IV non-Hodgkin’s lymphomas of favorable histologic types. Ann Intern Med 90:10-13, 1979.
26. Advani R, Rosenberg SA, Horning SJ: Stage I and II follicular non-Hodgkin’s lymphoma: Long term follow-up of no initial therapy. J Clin Oncol 22:1454-1459, 2004.
27. Hunault-Berger M, Ifrah N, Solal- Celigny P: Groupe Ouest-Est des Leucemies Aiguee et des Maladies du Sang (GOELAMS): Intensive therapies in follicular non-Hodgkin lymphomas. Blood 100:1141-1152, 2002.
28. Horning SJ: Follicular lymphoma: Have we made any progress? Ann Oncol 11:23-27, 2000.
29. Gospodarowicz MK, Bush RS, Brown TC, et al: Prognostic factors in nodular lymphomas: A multivariate analysis based on the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys 10:489-497, 1984.
30. Maartense E, Le Cessie S, Kluin- Nelemans HC, et al: Age-related differences among patients with follicular lymphoma and the importance of prognostic scoring systems: Analysis from a population-based non- Hodgkin’s lymphoma registry. Ann Oncol 13:1275-1284, 2002.
31. Paryani SB, Hoppe RT, Cox RS, et al: Analysis of non-Hodgkin’s lymphomas with nodular and favorable histologies, stages I and II. Cancer 52:2300-2307, 1983.
32. Denham JW, Denham E, Dear KB, et al: The follicular non-Hodgkin’s lymphomas-I. The possibility of cure. Eur J Cancer 32A:470- 479, 1996.
33. Aviles A, Delgado S, Fernandez R, et al: Combined therapy in advanced stages (III and IV) of follicular lymphoma increases the possibility of cure: Results of a large controlled clinical trial. Eur J Haematol 68:144-149, 2002.
34. Corradini P, Ladetto M, Zallio F, et al: Long-term follow-up of indolent lymphoma patients treated with high-dose sequential chemotherapy and autografting: Evidence that durable molecular and clinical remission frequently can be attained only in follicular subtypes. J Clin Oncol 22:1460-1468, 2004.
35. Ngan BY, Chen-Levy Z, Weiss LM, et al: Expression in non-Hodgkin’s lymphoma of the bcl-2 protein associated with the t(14;18) chromosomal translocation. N Engl J Med 318:1638-1644, 1988.
36. Schuler F, Hirt C, Dolken G: Chromosomal translocation t(14;18) in healthy individuals. Semin Cancer Biol 13:203-209, 2003.
37. Brice P, Bastion Y, Lepage E, et al: Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: A randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 15:1110- 1117, 1997.
38. Mac Manus MP, Hoppe RT: Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol 14:1282-1290, 1996.
39. Wilder RB, Jones D, Tucker SL, et al: Long-term results with radiotherapy for stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys 51:1219-1227, 2001.
40. Ott OJ, Rodel C, Gramatzki M, et al: Radiotherapy for stage I-III nodal low-grade non-Hodgkin’s lymphoma. Strahlenther Onkol 179:694-701, 2003.
41. Sack H, Hoederath A, Stuschke M, et al: Radiotherapy of follicle center lymphoma. Results of a German multicenter and prospective study. Members of the Study Group “NHL-early stages.” Strahlenther Onkol 174:178-185, 1998.
42. Taylor RE, Allan SG, McIntyre MA, et al: Low grade stage I and II non-Hodgkin’s lymphoma: Results of treatment and relapse pattern following therapy. Clin Radiol 39:287-290, 1988.
43. Pendlebury S, el Awadi M, Ashley S, et al: Radiotherapy results in early stage low grade nodal non-Hodgkin’s lymphoma. Radiother Oncol 36:167-171, 1995.
44. Vaughan Hudson B, Vaughan Hudson G, MacLennan KA, et al: Clinical stage I non- Hodgkin’s lymphoma: Long-term follow-up of patients treated by the British National Lymphoma Investigation with radiotherapy alone as initial therapy. Br J Cancer 69:1088-1093, 1994.
45. Neumann H, Blanck H, Koch R, et al: Follicle Centre Lymphoma: Treatment results for stage I and II. Strahlenther Onkol 179:840- 846, 2003.
46. Stuschke M, Hoederath A, Sack H, et al: Extended field and total central lymphatic radiotherapy in the treatment of early stage lymph node centroblastic-centrocytic lymphomas: Results of a prospective multicenter study. Study Group NHL-fruhe Stadien. Cancer 80:2273-2284, 1997.
47. McLaughlin P, Fuller LM, Velasquez WS, et al: Stage I-II follicular lymphoma. Treatment results for 76 patients. Cancer 58:1596- 1602, 1986.
48. Lawrence TS, Urba WJ, Steinberg SM, et al: Retrospective analysis of stage I and II indolent lymphomas at the National Cancer Institute. Int J Radiat Oncol Biol Phys 14:417- 424, 1988.
49. Soubeyran P, Eghbali H, Bonichon F, et al: Localized follicular lymphomas: Prognosis and survival of stages I and II in a retrospective series of 103 patients. Radiother Oncol 13:91-98, 1988.
50. Jeffery GM, Mead GM, Whitehouse JM, et al: Involved field radiotherapy or chemotherapy in the management of stage I nodal intermediate grade non-Hodgkin’s lymphoma. Br J Cancer 64:933-937, 1991.
51. Tezcan H, Vose JM, Bast M, et al: Limited stage I and II follicular non-Hodgkin’s lymphoma: The Nebraska Lymphoma Study Group experience. Leuk Lymphoma 34:273-285, 1999.
52. Yahalom J, Varsos G, Fuks Z, et al: Adjuvant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy after radiation therapy in stage I low-grade and intermediate- grade non-Hodgkin lymphoma. Results of a prospective randomized study. Cancer 71:2342-2350, 1993.
53. McLaughlin P, Fuller L, Redman J, et al: Stage I-II low-grade lymphomas: A prospective trial of combination chemotherapy and radiotherapy. Ann Oncol 2(suppl):137-140, 1991.
54. Seymour JF, Pro B, Fuller LM, et al: Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin’s lymphoma. J Clin Oncol 21:2115-2122, 2003.
55. Richards MA, Gregory WM, Hall PA, et al: Management of localized non-Hodgkin’s lymphoma: The experience at St. Bartholomew’s Hospital 1972-1985. Hematol Oncol 7:1-18, 1989.
56. Young RC, Longo DL, Glatstein E, et al: The treatment of indolent lymphomas: Watchful waiting v aggressive combined modality treatment. Semin Hematol 25(suppl 2):11-16, 1988.
57. Ardeshna KM, Smith P, Norton A, et al: British National Lymphoma Investigation. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: A randomised controlled trial. Lancet 362:516-522, 2003.
58. Hoppe RT, Kushlan P, Kaplan HS, et al: The treatment of advanced stage favorable histology non-Hodgkin’s lymphoma: A preliminary report of a randomized trial comparing single agent chemotherapy, combination chemotherapy, and whole body irradiation. Blood 58:592-598, 1981.
59. Kennedy BJ, Bloomfield CD, Kiang DT, et al: Combination versus successive single agent chemotherapy in lymphocytic lymphoma. Cancer 41:23-28, 1978.
60. Portlock CS, Rosenberg SA, Glatstein E, et al: Treatment of advanced non-Hodgkin’s lymphomas with favorable histologies: Preliminary results of a prospective trial. Blood 47:747- 756, 1976.61. Peterson BA, Petroni GR, Frizzera G, et al: Prolonged single-agent versus combination chemotherapy in indolent follicular lymphomas: A study of the Cancer and Leukemia Group B. J Clin Oncol 21:5-15, 2003.
62. Jones SE, Grozea PN, Metz EN, et al: Superiority of Adriamycin-containing combination chemotherapy in the treatment of diffuse lymphoma: A Southwest Oncology Group study. Cancer 43:417-425, 1979.
63. Ezdinli EZ, Costello WG, Silverstein MN, et al: Moderate versus intensive chemotherapy of prognostically favorable non- Hodgkin’s lymphoma: A progress report. Cancer 46:29-33, 1980.
64. Zinzani PL, Pulsoni A, Perrotti A, et al: Fludarabine plus mitoxantrone with and without rituximab versus CHOP with and without rituximab as front-line treatment for patients with follicular lymphoma. J Clin Oncol 22:2654-2661, 2004.
65. Price CG, Rohatiner AZ, Steward W, et al: Interferon alfa-2b in addition to chlorambucil in the treatment of follicular lymphoma: Preliminary results of a randomized trial in progress. Eur J Cancer 27(suppl 4):S34-S36, 1991.
66. Chisesi T, Congiu M, Contu A, et al: Randomized study of chlorambucil (CB) compared to interferon (alfa-2b) combined with CB in low-grade non-Hodgkin’s lymphoma: An interim report of a randomized study. Non- Hodgkin’s Lymphoma Cooperative Study Group. Eur J Cancer 27(suppl 4):S31-33, 1991.
67. Solal-Celigny P, Lepage E, Brousse N, et al: Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d’Etude des Lymphomes de l’Adulte. N Engl J Med 329:1608-1614, 1993.
68. Ozer H, Anderson JR, Peterson BA, et al: Combination trial of subcutaneous recombinant alpha 2b interferon and oral cyclophosphamide in follicular low-grade non-Hodgkin’s lymphoma. Med Pediatr Oncol 22:228-235, 1994.
69. Solal-Celigny P, Lepage E, Brousse N, et al: Doxorubicin-containing regimen with or without interferon alfa-2b for advanced follicular lymphomas: Final analysis of survival and toxicity in the Groupe d’Etude des Lymphomes Folliculaires 86 Trial. J Clin Oncol 16:2332- 2338, 1998.
70. Coiffier B, Neidhardt-Berard EM, Tilly H, et al: Fludarabine alone compared to CHVP plus interferon in elderly patients with follicular lymphoma and adverse prognostic parameters: A GELA study. Groupe d’Etudes des Lymphomes de l’Adulte. Ann Oncol 10:1191- 1197, 1999.
71. Rohatiner A, Radford J, Deakin D, et al: A randomized controlled trial to evaluate the role of interferon as initial and maintenance therapy in patients with follicular lymphoma. Br J Cancer 85:29-35, 2001.
72. Neri N, Aviles A, Cleto S, et al: Chemotherapy plus interferon-alpha 2b versus chemotherapy in the treatment of follicular lymphoma. J Hematother Stem Cell Res 10:669- 674, 2001.
73. Rohatiner AZ, Gregory W, Peterson B, et al: A meta-analysis (MA) of randomised trials evaluating the role of interferon (IFN) as treatment for follicular lymphoma (FL). Proc Am Soc Clin Oncol 17:4a, 1998.
74. McLaughlin P, Hagemeister FB, Swan F Jr, et al: Phase I study of the combination of fludarabine, mitoxantrone, and dexamethasone in low-grade lymphoma. J Clin Oncol 12:575- 579, 1994.
75. Solal-Celigny P, Brice P, Brousse N, et al: Phase II trial of fludarabine monophosphate as first-line treatment in patients with advanced follicular lymphoma: A multicenter study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 14:514-519, 1996.
76. Emmanouilides C, Rosen P, Rasti S, et al: Treatment of indolent lymphoma with fludarabine/mitoxantrone combination: A phase II trial. Hematol Oncol 16:107-116, 1998.
77. Zinzani PL, Magagnoli M, Moretti L, et al: Randomized trial of fludarabine versus fludarabine and idarubicin as frontline treatment in patients with indolent or mantle-cell lymphoma. J Clin Oncol 18:773-779, 2000.
78. Crawley CR, Foran JM, Gupta RK, et al: A phase II study to evaluate the combination of fludarabine, mitoxantrone and dexamethasone (FMD) in patients with follicular lymphoma. Ann Oncol 11:861-865, 2000.
79. Rohatgi N, LaRocca RV, Bard V, et al: Phase II trial of sequential therapy with fludarabine followed by cyclophosphamide, mitoxantrone, vincristine, and prednisone for low-grade follicular lymphomas. Am J Hematol 70:181-185, 2002.
80. Klasa R, Meyer R, Shustik C, et al: Fludarabine versus CVP in previously treated patients with progressive low grade non- Hodgkin’s lymphomas (lg-NHL) (abstract 28). Proc Am Soc Clin Oncol 18:9a, 1999.
81. Hainsworth JD. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma: Interim follow- up of a multicenter phase II trial. Semin Oncol 29(1 suppl 2):25-29, 2002.
82. Hainsworth JD, Litchy S, Burris HA 3rd, et al: Rituximab as first-line and maintenance therapy for patients with indolent non- Hodgkin’s lymphoma. J Clin Oncol 20:4261- 4267, 2002.
83. Colombat P, Salles G, Brousse N, et al: Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood 97:101- 106, 2001.
84. Foran JM, Gupta RK, Cunningham D, et al: A UK multicentre phase II study of rituximab (chimaeric anti-CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. Br J Haematol 109:81-88, 2000.
85. Jaeger G, Neumeister P, Brezinschek R, et al: Rituximab (anti-CD20 monoclonal antibody) as consolidation of first-line CHOP chemotherapy in patients with follicular lymphoma: A phase II study. Eur J Haematol 69:21-26, 2002.
86. Maloney DG, Press OW, Braziel RM, et al: A phase II trial of CHOP followed by rituximab chimeric monoclonal anti-CD20 antibody for treatment of newly diagnosed follicular non-Hodgkin’s lymphoma: SWOG 9800 (abstract 3502). Blood 98:843a, 2001.
87. Czuczman MS, Grillo-Lopez AJ, LoBuglio AI, et al: Patients with low-grade NHL treated with rituximab + CHOP experience prolonged clinical and molecular remission (abstract 1493). Blood 102:411a, 2003.
88. Hiddemann W, Dreyling MH, Forstpointner R, et al: Combined immuno-chemotherapy (R-CHOP) significantly improves time to treatment failure in first line therapy of follicular lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) (abstract 352). Blood 102:104a, 2003.
89. Marcus R, Imrie K, Belch A, et al: An international multi-centre, randomized, openlabel, phase III trial comparing rituximab added to CVP chemotherapy to CVP chemotherapy alone in untreated stage III/IV follicular non- Hodgkin’s lymphoma (abstract 87). Blood 102:28a, 2003.
90. Witzig TE, Gordon LI, Cabanillas F, et al: Randomized controlled trial of yttrium- 90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 20:2453-2463, 2002.
91. Wiseman GA, Gordon LI, Multani PS, et al: Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: A phase II multicenter trial. Blood 99:4336-4342, 2002.
92. Witzig TE, Flinn IW, Gordon LI, et al: Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol 20:3262-3269, 2002.
93. Vose JM, Bierman PJ, Lynch JC, et al: Phase I clinical trial of Zevalin (90Yibritumomab) in patients with B-cell non- Hodgkin’s lymphoma (NHL) with relapsed disease following high-dose chemotherapy and autologous stem cell transplantation (ASCT) (abstract 92). Blood 102:30a, 2003.
94. Vose JM, Wahl RL, Saleh M, et al: Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade Bcell non-Hodgkin’s lymphomas. J Clin Oncol 18:1316-1323, 2000.
95. Kaminski MS, Estes J, Zasadny KR, et al: Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: Updated results and long-term follow-up of the University of Michigan experience. Blood 96:1259-1266, 2000.
96. Kaminski MS, Zelenetz AD, Press OW, et al: Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol 19:3918-3928, 2001.
97. Coleman M, Kaminski MS, Knox SJ, et al: The BEXXAR therapeutic regimen (tositumomab and iodine I 131 tositumomab) produced durable complete remissions in heavily pretreated patients with non-Hodgkin s lymphoma (NHL), rituximab-relapsed/refractory disease, and rituximab-naive disease (abstract 89). Blood 102:29a, 2003.
98. Davies AJ, Rohatiner AZ, Howell S, et al: Tositumomab and iodine I 131 tositumomab for recurrent indolent and transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 22:1469-1479, 2004.
99. Press OW, Unger JM, Braziel RM, et al: A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood 102:1606- 1612, 2003.
100. Kaminski M, Estes J, Tuck M, et al: Iodine I 131 Tositumomab therapy for previously untreated follicular lymphoma (FL) (abstract 11). Proc Am Soc Clin Oncol 19:5a, 2000.
101. Colombat P, Binet C, Linassier C, et al: High dose chemotherapy with autologous marrow transplantation in follicular lymphomas. Leuk Lymphoma 7(suppl):3-6, 1992.
102. Rohatiner AZ, Freedman A, Nadler L, et al: Myeloablative therapy with autologous bone marrow transplantation as consolidation therapy for follicular lymphoma. Ann Oncol 5(suppl 2):143-146, 1994.
103. Colombat P, Donadio D, Fouillard L, et al: Value of autologous bone marrow transplantation in follicular lymphoma: A France Autogreffe retrospective study of 42 patients. Bone Marrow Transplant 13:157-162, 1994.
104. Bastion Y, Brice P, Haioun C, et al: Intensive therapy with peripheral blood progenitor cell transplantation in 60 patients with poorprognosis follicular lymphoma. Blood 86:3257-3262, 1995.
105. Haas R, Moos M, Mohle R, et al: Highdose therapy with peripheral blood progenitor cell transplantation in low-grade non- Hodgkin’s lymphoma. Bone Marrow Transplant 17:149-155, 1996.
106. Bociek RG, Bierman PJ, Lymch JC, et al: High-dose therapy with autologous hematopoietic stem cell transplantation for follicular non-Hodgkin’s lymphoma: Long term results (abstract 8). Proc Am Soc Clin Oncol 18:3a, 1999.
107. Foran JM, Apostolidis J, Papamichael D, et al: High-dose therapy with autologous haematopoietic support in patients with transformed follicular lymphoma: A study of 27 patients from a single centre. Ann Oncol 9:865- 869, 1998.
108. Lopez R, Martino R, Sureda A, et al: Autologous stem cell transplantation in advanced follicular lymphoma. A single center experience. Haematologica 84:350-355, 1999.
109. Freedman AS, Neuberg D, Mauch P, et al: Long-term follow-up of autologous bone marrow transplantation in patients with relapsed follicular lymphoma. Blood 94:3325-3333, 1999.
110. Apostolidis J, Gupta RK, Grenzelias D, et al: High-dose therapy with autologous bone marrow support as consolidation of remission in follicular lymphoma: Long-term clinical and molecular follow-up. J Clin Oncol 18:527-536, 2000.
111. Brice P, Simon D, Bouabdallah R, et al; Groupe d’Etude des Lymphomes de l’Adulte (GELA): High-dose therapy with autologous stem-cell transplantation (ASCT) after first progression prolonged survival of follicular lymphoma patients included in the prospective GELF 86 protocol. Ann Oncol 11:1585-1590, 2000.
112. Horning SJ, Negrin RS, Hoppe RT, et al: High-dose therapy and autologous bone marrow transplantation for follicular lymphoma in first complete or partial remission: Results of a phase II clinical trial. Blood 97:404-409, 2001.
113. Ladetto M, Corradini P, Vallet S, et al: High rate of clinical and molecular remissions in follicular lymphoma patients receiving highdose sequential chemotherapy and autografting at diagnosis: A multicenter, prospective study by the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Blood 100:1559-1565, 2002.
114. Recher C, Huynh A, Huguet F, et al: Tandem transplant for follicular lymphoma in patients under 60 years (abstract 871). Blood 102:248a, 2003.
115. Gopal AK, Gooley TA, Maloney DG, et al: High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: A multivariable cohort analysis. Blood 102:2351- 2357, 2003.
116. van Besien K, Loberiza FR Jr, Bajorunaite R, et al: Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood 102:3521-3529, 2003.
117. Schouten HC, Qian W, Kvaloy S, et al: High-dose therapy improves progression-free survival and survival in relapsed follicular non- Hodgkin’s lymphoma: Results from the randomized European CUP trial. J Clin Oncol 21:3918-3927, 2003.
118. van Besien K, Sobocinski KA, Rowlings PA, et al: Allogeneic bone marrow transplantation for low-grade lymphoma. Blood 92:1832-1836, 1998.
119. Juckett M, Rowlings P, Hessner M, et al: T cell-depleted allogeneic bone marrow transplantation for high-risk non-Hodgkin’s lymphoma: Clinical and molecular follow-up. Bone Marrow Transplant 21:893-899, 1998.
120. Stein RS, Greer JP, Goodman S, et al: High-dose therapy with autologous or allogeneic transplantation as salvage therapy for small cleaved cell lymphoma of follicular center cell origin. Bone Marrow Transplant 23:227-233, 1999.
121. Khouri IF, Saliba RM, Giralt SA, et al: Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: Low incidence of toxicity, acute graft-versus-host disease, and treatmentrelated mortality. Blood 98:3595-3599, 2001.
122. Forrest DL, Thompson K, Nevill TJ, et al: Allogeneic hematopoietic stem cell transplantation for progressive follicular lymphoma. Bone Marrow Transplant 29:973-978, 2002.
123. Yakoub-Agha I, Fawaz A, Folliot O, et al: Allogeneic bone marrow transplantation in patients with follicular lymphoma: A single center study. Bone Marrow Transplant 30:229- 234, 2002.
124. Ho AY, Devereux S, Mufti GJ, et al: Reduced- intensity rituximab-BEAM-CAMPATH allogeneic haematopoietic stem cell transplantation for follicular lymphoma is feasible and induces durable molecular remissions. Bone Marrow Transplant 31:551-557, 2003.
125. Verdonck LF, Dekker AW, Lokhorst HM, et al: Allogeneic versus autologous bone marrow transplantation for refractory and recurrent low grade non-Hodgkin’s lymphoma. Blood 90:4201-4205, 1997.
126. Deshpande AT, Bociek RG, Bierman PJ, et al: Long term outcome following autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Biol Blood Marrow Transplant 10(suppl):28, 2004.
127. van Besien KW, de Lima M, Giralt SA, et al: Management of lymphoma recurrence after allogeneic transplantation: The relevance of graft-versus-lymphoma effect. Bone Marrow Transplant 19:977-982, 1997.
128. Mandigers CM, Verdonck LF, Meijerink JP, et al: Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant 32:1159-1163, 2003.
129. Hsu FJ, Caspar CB, Czerwinski D, et al: Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma: Long-term results of a clinical trial. Blood 89:3129-3135, 1997.
130. Timmerman JM, Czerwinski DK, Davis TA, et al: Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: Clinical and immune responses in 35 patients. Blood 99:1517- 1526, 2002.
131. Vose JM, Chiu BC, Cheson BD, et al: Update on epidemiology and therapeutics for non-Hodgkin’s lymphoma. Hematology (Am Soc Hematol Educ Program) pp 241-262, 2002.
132. Leonard JP, Coleman M, Ketas JC, et al: Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol 21:3051-3059, 2003.
133. Postema EJ, Raemaekers JM, Oyen WJ, et al: Final results of a phase I radioimmunotherapy trial using (186) Re-epratuzumab for the treatment of patients with non-Hodgkin’s lymphoma. Clin Cancer Res 9:3995S-4002S, 2003.
134. Gingrich RD, Dahle CE, Hoskins KF, et al: Identification and characterization of a new surface membrane antigen found predominantly on malignant B lymphocytes. Blood 75:2375-2387, 1990.
135. Press OW, Leonard JP, Coiffier B, et al: Immunotherapy of non-Hodgkin’s lymphomas. Hematology (Am Soc Hematol Educ Program) 221-240, 2001.
136. Link BK, Wang H, Byrd JC, et al: Phase I study of Hu1D10 monoclonal antibody in patients with B-cell lymphoma (abstract 1135). Proc Am Soc Clin Oncol 20:284a, 2001.
137. Czuczman M, Witzig TE, Vose JM, et al: Results of a phase I/II multicenter trial of galiximab (IDEC-114, anti-CD80 antibody) therapy for relapsed or refractory follicular lymphoma (abstract 2393). Blood 102:647a-648a, 2003.
138. Gordon LI, Moore JO, Cheson BD, et al: Phase I results from a multicenter trial of galiximab (anti-CD80 Antibody, IDEC-114) in combination with rituximab for the treatment of follicular lymphoma (abstract 4951). Blood 102:307b, 2003.
139. Webb A, Cunningham D, Cotter F, et al: BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet 349:1137- 1141, 1997.
140. Freedman AS, Friedberg JW, Mauch PM, et al: Non-Hodgkin’s Lymphomas, pp 367- 388. Philadelphia, Lippincott Williams & Wilkins, 2003.
141. Waters JS, Webb A, Cunningham D, et al: Phase I clinical and pharmacokinetic study of bcl-2 antisense oligonucleotide therapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol 18:1812-1823, 2000.
142. Kisselev AF, Goldberg AL: Proteasome inhibitors: From research tools to drug candidates. Chem Biol 8:739-758, 2001.
143. Orlowski RZ, Eswara JR, Lafond- Walker A, et al: Tumor growth inhibition induced in a murine model of human Burkitt’s lymphoma by a proteasome inhibitor (abstract). Cancer Res 58: 4342-4348, 1998.
144. O’Connor O, Wright J, Moskowitz CH, et al: Promising activity of the proteasome inhibitor bortezomib (Velcade) in the treatment of indolent non-Hodgkin’s lymphoma and mantle cell lymphoma (abstract 2346). Blood 102:636a, 2003.
145. Warren TL, Dahle CE, Weiner GJ: CpG oligodeoxynucleotides enhance monoclonal antibody therapy of a murine lymphoma. Clin Lymphoma 1:57-61, 2000.
146. Friedberg JW, Kim H, McCauley M, et al: Combination immunotherapy for non- Hodgkin’s lymphoma (NHL) with CpG oligonucleotide (1018 ISS) and rituximab: Biologic responses through interferon-alpha/beta-inducible gene expression without significant toxicity (abstract 232). Blood 102:69a, 2003.
147. Chopra R, Goldstone AH, Pearce R, et al. Autologous versus allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma: A case-controlled analysis of the European Bone Marrow Transplant Group registry data. J Clin Oncol 10:1690-1695, 1992.
148. Khouri IF, Saliba RM, Giralt SA, et al: Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: Low incidence of toxicity, acute graft-versus-host disease, and treatmentrelated mortality. Blood 98:3595-3599, 2001.