Commentary (Harding/Bow): Infectious Complications of Lung Cancer
Lung cancer is the most commoncause of cancer-relatedmortality in the United Statesand worldwide.[1] In the UnitedStates, lung cancer was responsiblefor an estimated 160,440 deaths in2004. This surpassed the combinedmortality resulting from colorectal,breast, and prostate cancer.
Lung cancer is the most common cause of cancer-related mortality in the United States and worldwide.[1] In the United States, lung cancer was responsible for an estimated 160,440 deaths in 2004. This surpassed the combined mortality resulting from colorectal, breast, and prostate cancer. Infections are a major cause of morbidity and mortality in all cancers. A wide array of tumor-, host-, and treatment- related factors place cancer patients at high risk of infection. In this issue of ONCOLOGY, Dr. Seo reviews these factors with regard to specific infections and syndromes including those related to treatment-related myelosuppression and immunosupression. The many advances in lung cancer therapy have led to increasingly complicated forms of treatment involving surgery, chemotherapy, and radiation. Many newer treatment protocols not only use chemotherapy and radiation concurrently but advocate the initiation of therapy soon after surgery. This approach places more stress on host defense mechanisms. Impact of Adjuvant Therapy
The most recent advance in the treatment of lung cancer has been in the administration of multiagent chemotherapy in the adjuvant setting of earlystage non-small-cell lung cancer. The rationale for adjuvant chemotherapy was based on observations in animal models wherein subjects with smaller tumor burdens were characterized by a higher proportion of dividing cells, a smaller likelihood of developing drug resistance, and a higher treatment-related survival rate.[2] As has been clearly shown in both breast cancer and colon cancer, adjuvant chemotherapy after surgical intervention can significantly decrease risk of recurrence and death by addressing residual microscopic disease at primary and distant sites after surgery.[3] Until recently, however, there has been a paucity of data showing the benefits and possible harms of adjuvant chemotherapy in non- small-cell lung cancer. There have been nine major randomized trials studying adjuvant chemotherapy vs observation alone in early non-small-cell lung cancer.[4-12] All of the early trials failed to show any survival advantage.[4-8] They did, however, highlight some of the issues that Dr. Seo addresses with regard to lung cancer patients being prone to infection due to chemotherapy-related toxicities. Not only do these treatments disrupt the host's physical defenses in terms of mucosal breakdown within the gastrointestinal, sinopulmonary, and genitourinary tracts, but the associated myelotoxicity can also have a significant impact. These early trials showed a rather wide range of grade 4 (life-threatening) neutropenia rates-from 7% to 60%-in the chemotherapy arm (Table 1). These trials, however, did not report on the incidence of related fever and infection. Almost 40 years ago, investigators observed the relationship between the degree and duration of neutropenia caused by chemotherapy and the risk and severity of infectious complications in cancer patients.[13] Adjuvant chemotherapy in non-small-cell lung cancer was not accepted as standard of care until more recently reported trials.[14] Two large well designed randomized trials in this area were reported at last year's annual meeting of the American Society of Clinical Oncology.[ 11,12] Both of these studies demonstrated a significant large survival advantage with the use of adjuvant chemotherapy in early-stage non- small-cell lung cancer, and these data are leading to a new standard of care in North America. However, these trials also reported a significant amount of myelotoxicity (Table 1).[11,12] Although one trial reported a 7% rate of febrile neutropenic episodes (and there was one report of a septic death secondary to chemotherapy), the risk and spectrum of adjuvant treatment-related infectious complications has yet to be described in this setting. A recent published meta-analysis assessing adjuvant chemotherapy in 11 trials of resected non-small-cell lung cancer further demonstrated the survival benefit associated with therapy.[15] Of the 5,716 patients included in the analysis, 2,873 patients received adjuvant chemotherapy. While 90% of the patients that received chemotherapy were reported to have demonstrated some treatment-related toxicity, the analysis showed a grade 4 neutropenia rate of 14%. No data were reported on the incidence of fever, neutropenia and infections. Economic Effect
The treatment-related toxicities and infectious complications associated with adjuvant chemotherapy in non- small-cell lung cancer can have significant economic impact. For instance, in the year 2000, Statistics Canada reported 11,404 new primary lung cancer diagnoses in Canada.[16] Roughly 30% of these patients undergo surgical resection for primary treatment.[14] It is projected today in the oncology community that another 60% of these patients will be given adjuvant chemotherapy for their disease, implying that over 2,000 new patients per year in Canada will be subjected to the toxicity of chemotherapy and the risk of infectious complications.
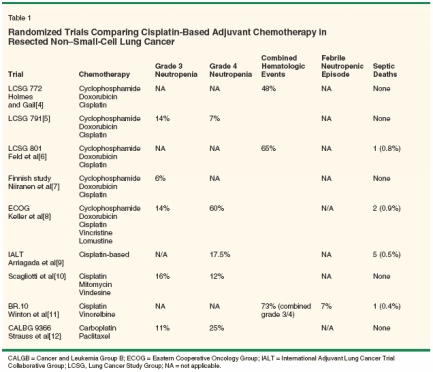
Based on the recent BR.10 data,[11] this could mean that at least 143 patients could have one or more episodes of infection. Although most of these episodes may be classifiable as "low risk" according to the Multinational Association for Supportive Care in Cancer,[ 17] this will result in an increase in the use of antibiotics, possibly the increased use of granulocyte colony-stimulating factor (G-CSF [Neupogen]), and increased hospital admission, as summarized in the latest guidelines regarding fever and neutropenia published by the National Comprehensive Cancer Network.[18] This increases the economic burden on hospitals, cancer agencies, and patients. Decision analysis has been used to estimate the direct medical, indirect medical, and time costs associated with febrile neutropenic episodes.[19] The direct costs include inpatient or outpatient facility costs, pharmaceuticals, diagnostic tests, procedures, and medical and nonmedical professional fees. The indirect and time costs that directly affect the patient's economic situation include the extra visits to the hospital, time off work, and possible loss of earning potential due to disability or premature death. One study estimated the range of inpatient and outpatient costs of neutropenia with and without infection to be $2,228 to $15,876 per episode (in US dollars).[20] Another study examining the costs of oral and intravenous antimicrobial therapy administered in outpatients and inpatients reported a range of $7,394 to $9,728 per episode.[21] Predicting Risk
The review by Dr. Seo helps the clinical oncologist to understand the circumstances under which infection risk can be predicted in lung cancer patients undergoing active treatment and supportive care. It appears that adjuvant chemotherapy in early non- small-cell lung cancer will become an additional circumstance wherein infection risk is increased. Ethical standards demand that patients be informed of these acute and potentially life-threatening risks. This is often a difficult dilemma in the adjuvant setting when there is no measurable cancer to gauge the success of the treatment. The published literature is at best incomplete with regard to the incidence of febrile neutropenic episodes and the potential risk for the increasing number of patients exposed to these treatment strategies. It is our duty as investigators to advocate the collection, analysis, and reporting of infectious complications in lung cancer patients in order to enhance our ability to counsel our patients about these risks and enhance the informed-consent process.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004.
2. Skipper HE, Schabel FM Jr, Wilcox WS: Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep 35:1-111, 1964.
3. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) Investigators:Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 345:939- 944, 1995.
4. Holmes EC, Gail M: Surgical adjuvant therapy for stage II and stage III adenocarcinoma and large-cell undifferentiated carcinoma. J Clin Oncol 4:710-715, 1986.
5. Lung Cancer Study Group: The benefit of adjuvant treatment for resected locally advanced non-small-cell lung cancer. J Clin Oncol 6:9-17, 1988.
6. Feld R, Rubinstein L, Thomas PA: Adjuvant chemotherapy with cyclophosphamide, doxorubicin, and cisplatin in patients with completely resected stage I non-small-cell lung cancer. The Lung Cancer Study Group. J Natl Cancer Inst 85:299-306, 1993.
7. Niiranen A, Niitamo-Korhonen S, Kouri M, et al: Adjuvant chemotherapy after radical surgery for non-small-cell lung cancer: A randomized study. J Clin Oncol 10:1927-1932, 1992.
8. Keller SM, Adak S, Wagner H, et al: A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med 343:1217-1222, 2000.
9. Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351-360, 2004.
10. Scagliotti GV, Fossati R, Torri V, et al: Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA nonsmall- cell lung cancer. J Natl Cancer Inst 95:1453-1461, 2003.
11. Winton TL, Livingston R, Johnson D, et al: Prospective randomised trial of adjuvant vinorelbine (VIN) and cisplatin (CIS) in completely resected stage IB and II non small cell lung cancer (NSCLC). Intergroup JBR.10 (abstract 7018). Proceedings of the Annual Meeting of the American Society of Clinical Oncology, New Orleans, June 5–8, 2004, latebreaking abstracts.
12. Strauss GM, Herndon J, Maddaus MA, et al: Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in stage IB non-small cell lung cancer (NSCLC): Report of Cancer and Leukemia Group B (CALGB) Protocol 9633 (abstract 7019). Proceedings of the Annual Meeting of the American Society of Clinical Oncology, New Orleans, June 5–8, 2004, late-breaking abstracts.
13. Bodey GP: The treatment of febrile neutropenia: From the Dark Ages to the present. Support Care Cancer 5:351-357, 1997.
14. Blum RH: Adjuvant chemotherapy for lung cancer-a new standard of care. N Engl J Med 350:404-405, 2004.
15. Hotta K, Matsuo K, Ueoka H, et al: Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: Reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol 22:3860-3867, 2004.
16. Statistics Canada: Canadian Cancer Registry Database, 2000. Available at www.statcan.ca/english/Pgdb/hlth61.htm. Accessed January 10, 2005.
17. Klastersky J, Paesmans M, Rubenstein EB, et al: The Multinational Association for Supportive Care in Cancer Risk Index: A multinational scoring system for identifying lowrisk febrile neutropenic cancer patients. J Clin Oncol 18:3038-3051, 2000.
18. Freifeld AG, Baden LR, Brown AE, et al: Fever and neutropenia. JNCCN 2:390-432, 2004.
19. Elting LS, Cantor SB: Outcomes and costs of febrile neutropenia: Adventures in the science and art of treatment choices. Support Care Cancer 10:189-196, 2002.
20. Elting LS, Shih YC: The economic burden of supportive care of cancer patients. Support Care Cancer 12:219-226, 2004.
21. McNabb J, Freifeld AG, Yee GC, et al: Economic outcomes of outpatient treatment of low-risk febrile neutropenia during cancer chemotherapy (abstract #815). Proceedings of the 39th Annual Meeting of the Infectious Diseases Society of America, San Francisco, October 25- 28, 2001.