Gemcitabine active as adjuvant Rx for pancreatic head ca
Last month, ONI reported evidence from two retrospective studies and one phase II trial for the use of adjuvant chemoradiation in resected pancreatic cancer patients (Feb. 2008, pages 1 and 31). Now, RTOG investigators report a strong trend toward improved survival in patients with resected cancer of the pancreatic head with gemcitabine (Gemzar) plus fluorouracil (5-FU)-based chemoradiation, compared with standard 5-FU chemoradiation (JAMA 299:1019-1026, 2008).

Last month, ONI reported evidence from two retrospective studies and one phase II trial for the use of adjuvant chemoradiation in resected pancreatic cancer patients (Feb. 2008, pages 1 and 31). Now, RTOG investigators report a strong trend toward improved survival in patients with resected cancer of the pancreatic head with gemcitabine (Gemzar) plus fluorouracil (5-FU)-based chemoradiation, compared with standard 5-FU chemoradiation (JAMA 299:1019-1026, 2008). “The RTOG 97-04 study represents the first US cooperative group adjuvant pancreatic phase III trial in 3 decades,” said principal investigator William Regine, MD, of the University of Maryland.
A total of 451 patients with resected pancreatic adenocarcinoma were evaluable, including 388 with pancreatic head tumors. A primary endpoint of the study was survival in patients with pancreatic head tumors, Dr. Regine said.
Patients were randomized to receive chemotherapy with either 5-FU, 250 mg/m2/d continuous infusion for 3 weeks, or gemcitabine, 30-minute infusion of 1,000 mg/m2 once weekly for 3 weeks, prior to chemoradiation therapy (50.4 Gy with a continuous infusion of 250 mg/m2 of 5-FU daily through radiation therapy) and for 12 weeks after chemoradiation.
Patients were stratified according to tumor diameter, nodal status, and surgical margins. All surviving patients were followed for a minimum of 4.1 years.
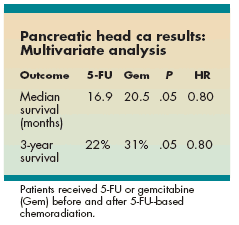
Dr. Regine reported no overall survival advantage for gemcitabine, compared with 5-FU, when all patients were analyzed (median follow-up 1.5 years for all patients and 4.7 years for surviving patients). For the pancreatic head cancer patients, there was a trend for improved median survival, with an increase in survival of about 4 months in the gemcitabine group, compared with 5-FU. In multivariate analysis, the advantage for gemcitabine was strengthened (P = .05, HR 0.80) (see Table).
Hematologic toxicity was significantly increased with gemcitabine: Grade 3 toxicity, 58% vs 9% for 5-FU, and grade 4 toxicity, 14% vs 1% (P < .001), but there was no difference in febrile neutropenia or infection, and no difference in the ability to complete chemotherapy or radiation therapy (> 85% in each group). Nonhematologic adverse events were similar in both arms.
The researchers commented that the results compare favorably with other phase III trials of chemoradiation in pancreatic head cancer (GITSG, EORTC, ESPAC-1, and CONKO-001) despite having a population of patients with more advanced disease than these other trials. In addition, Dr. Regine told ONI, the local recurrence rate of 23% in RTOG 97-04 is the lowest reported of any trial to date.
“This study will change standard practice across the country for postoperative treatment of this type of pancreatic cancer,” Dr. Regine maintained.
He noted that 70% of patients in both arms of the study had distant disease relapse. “Clearly, metastatic disease is a huge problem, and we need more clinical research to identify new systemic or targeted therapies to prevent this type of recurrence,” Dr. Regine said. RTOG researchers are planning to test new agents, using the new gemcitabine, 5-FU, radiation combination as the standard.